In the Scheetz household, back-to-school anxiety reached new heights this fall.
Jami Scheetz’s 15-year-old son Devon, who has severe asthma, kicked a brutal vaping habit over the summer, with help from a nicotine patch. But as soon as school started and he was once again around kids vaping, his habit returned. On Sept. 12, Devon vaped at school and immediately began sweating and vomiting. Though Scheetz, who lives in Sellersville, Pa., says her son is now fine, she can’t shake thoughts of kids who have been hospitalized or died after using e-cigarettes. “Vaping scares me more [than smoking], because they don’t know what’s really in it,” she says.
To a remarkable degree, a single company is front and center in one of the biggest public-health crises facing the country: the sharp rise in vaping among teenagers and young adults. In 2018, 30% of the nation’s 12th-graders reported vaping nicotine at least once in the past year, according to a January 2019 study sponsored by the National Institute on Drug Abuse. The study said the increase in vaping last year was “the largest ever recorded for any substance in the 44 years” that it has tracked adolescent drug use.

Though Juul is not the only e-cigarette for sale in the U.S., it is largely blamed for the vaping explosion and controls about 50% of the market, putting a sharp focus on the company. On Sept. 9, the Food and Drug Administration sent Juul a warning letter accusing the company of violating federal regulations by promoting its e-cigarettes as a safer option than traditional cigarettes and threatening the company with fines and product seizures if it continued. Two days later, the Trump Administration said it planned to pull from the market flavored e-cigarettes such as Juul’s mango, creme and mint pods. In the Oval Office, with First Lady Melania at his side, President Trump said, “We can’t allow people to get sick. And we can’t have our youth be so affected.” He added that the First Lady, who tweeted a warning about vaping, feels “very, very strongly” about the issue because of their teenage son Barron. Just days later, New York banned most flavored e-cigarettes statewide, following in the footsteps of Michigan and Juul’s home city of San Francisco, whose mayor signed an ordinance effectively banning e-cigarettes. The recent moves were prompted by U.S. Centers for Disease Control and Prevention (CDC) reports of almost 400 serious lung illnesses and six deaths it linked to vaping, which a congressional committee is also investigating. While Juul products have not been implicated in the deaths, the CDC in September advised Americans to “consider not using e-cigarette products” while its investigation is ongoing. The American Lung Association went further, saying in a statement that “no one should use e-cigarettes or any other tobacco product.” Huge international markets, including India and China, are also restricting the sale of e-cigarettes.
Given the possible risks to the nation’s youth, Juul’s rapid growth has been accompanied by remarkably little oversight or regulation. And while there is a legitimate debate over whether e-cigarettes are safer for adult smokers than traditional cigarettes, and whether they can help addicts quit smoking, critics argue that Juul has assiduously followed Big Tobacco’s playbook: aggressively marketing to youth and making implied health claims a central pillar of its business plan. Juul maintains that it is not Big Tobacco 2.0. In eight months, unless e-cigarette companies can prove to the FDA that vaping is “appropriate for the protection of public health,” the products could be pulled from the market. That would curtail youth use, but some fear it could also cut off adult smokers’ access to a potentially beneficial product.

Juul, which was valued at $38 billion by its investors before the Trump Administration’s crackdown, is now facing what CEO Kevin Burns in July called an “existential” threat, due to rising levels of youth use. Lobbyists, staff scientists and PR experts are working feverishly to respond to the growing public outrage. “Sh-t happens,” Burns told TIME in July, foreshadowing the rocky summer to come. “We’ve got to respond. I would love it to be less dynamic here than it is, because it’s not easy on the organization. But I think the organization understands that we’re at the forefront here and it’s going to be volatile.” Juul says that it does not make health claims and that it has never marketed to youth. The company has taken recent steps to make it harder for young people to illegally buy its products, both online and in stores.
Nobody hates Juul more than parents, many of whom are watching their children fall prey to the “epidemic on speed” that is Juuling, as New York parent Erin Mills puts it. She blames her son’s two-year addiction to Juuls for causing his grades and social life to plummet, while she says she and her husband watched helplessly. It’s “like this tsunami,” she says, “and I see my child going under.”
To help parents like Mills, New York City mothers Meredith Berkman, Dorian Fuhrman and Dina Alessi formed the advocacy group Parents Against Vaping E-Cigarettes in 2018. It has grown to about a dozen chapters across the country. Berkman argued at a congressional hearing in July that today’s kids are becoming “an entire generation of nicotine addicts” and “human guinea pigs for the Juul experiment.” Filmmaker Judd Apatow made his opinion clear on Sept. 9, tweeting, “Juul is some evil sh-t … Keep your kids away from it. It’s a scam to get you addicted.”
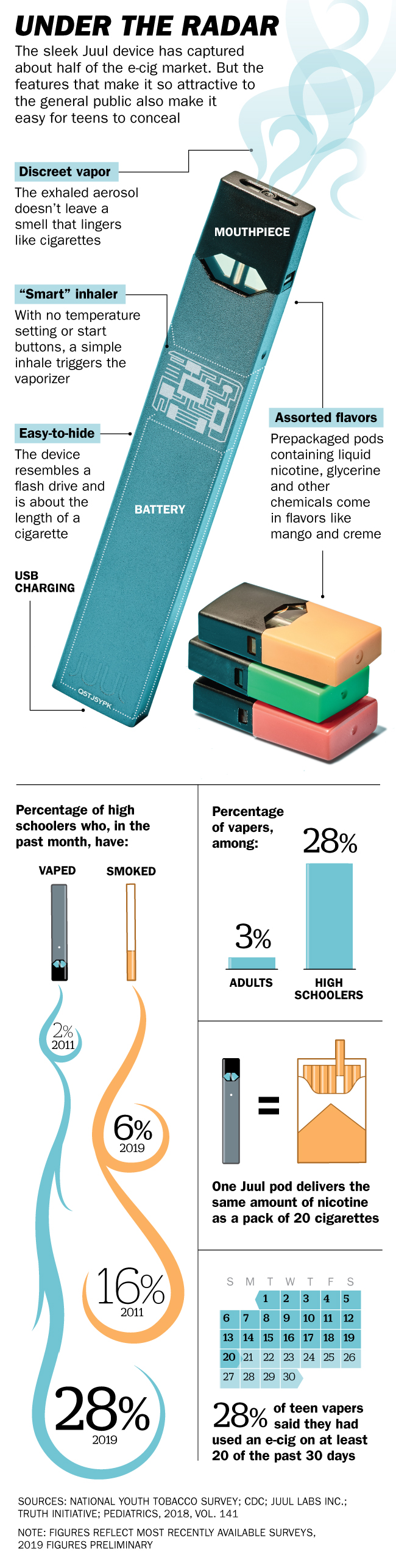
Hundreds of U.S. school districts have installed electronic vape detectors in their bathrooms–or “Juul rooms”–and one in Alabama went further, removing some bathroom doors to make it harder to vape in secret. But the product’s design has complicated that task. Juul’s $35 sleek slate gray and silver e-cigs are often compared to flash drives or iPhones, in sharp contrast to the clunky, tank-style devices that preceded them. They’re small enough to fit in the palm of your hand and subtly vaporize pods of liquid containing nicotine, flavorings and other chemicals. A four-pack costs $16, and each 200-puff pod delivers as much nicotine as a pack of 20 cigarettes.
Halving cigarette-smoking rates since the 1960s remains one of America’s biggest public-health triumphs, even though smoking–which is responsible for more than 480,000 deaths annually–remains the leading cause of preventable death in the U.S. Teen cigarette smoking, too, had seen historic declines in recent years. Now that hard-won success may be in peril.
The magnitude of the teen-vaping problem began to emerge last November, when the FDA announced that almost 21% of high school students had vaped during the previous month, a 78% increase over the year before. That number jumped again this year, to 27.5%, meaning that more than 4 million American teenagers vape regularly, according to preliminary reports from federal health officials. The 2018 National Youth Tobacco Survey found that about 3.5% of high school students–more than 525,000 teenagers–vaped every or almost every day. Particularly alarming is vaping’s appeal to younger teenagers. Use among eighth-graders more than doubled in 2018, to 10%, according to data posted by the Department of Health and Human Services (HHS). There are concerns that such early adoption of vaping will “represent a gateway to the use of traditional cigarettes,” according to HHS. Eighth-graders who vape are 10 times as likely to eventually smoke cigarettes as their nonvaping peers, HHS says.
E-cigs had been on the market for almost a decade before Juul–competitors today include Blu and NJOY–though none had really taken off. Juul, which made an estimated $1.27 billion during the first half of this year, sold 2.2 million devices in 2016, its first full year on the market, and 16.2 million the year after, according to CDC data. Today Juul is a major part of the pop-culture zeitgeist, with flourishing hashtags on Instagram and Twitter (#Juul, #JuulTricks, #JuulMemes) and accounts devoted to celebrity Juul use (@Sophie_Turner_Juuling).
For young people, the relationship between vaping and taking up smoking is murky. The percentage of high schoolers smoking cigarettes rose from 7.6% to 8.1% in 2018. But so far this year, even as vaping has continued to soar, youth smoking rates dropped back down to 5.8%, according to HHS data. Still, many fear that vaping is creating lifelong nicotine addicts. “They’re bringing kids who are at low risk of smoking into the margin,” says Stanton Glantz, a professor of medicine at the University of California, San Francisco (UCSF). “A lot of those kids then transition to regular cigarettes.” Just 20 years ago, 23% of 12th-graders smoked daily, compared with 3.6% in 2018. With youth nicotine use ticking up because of vaping, history seems in danger of repeating itself.
Juul Co-founders James Monsees, 39, and Adam Bowen, 44, didn’t set out to create America’s most hated startup. As graduate students in product design at Stanford 14 years ago, they dreamed up the device that would disrupt a global industry and become a status symbol for many young people. In 2018, Altria (the parent company of brands including Marlboro) bought a 35% stake for $12.8 billion, making Monsees and Bowen, who each own less than 5% of the company, worth more than $1 billion each.

Monsees, a physics and studio-art graduate of Kenyon College, and Bowen, who studied physics at Pomona College, famously became friends during smoke breaks at Stanford. It was their own struggle to quit that inspired them to create a product that could help. In 2007 they founded Ploom Inc., which would later be known as Pax Labs. At Pax, they began developing a line of cannabis vaporizers and the nicotine-vaporizing device that would become Juul. As the company ramped up ahead of Juul’s 2015 launch, Monsees and Bowen–who were named to TIME’s 2019 list of 100 most influential people–began making moves that didn’t fit so neatly into the public-health-warrior narrative they’d honed. At the congressional hearing in July, Stanford tobacco-advertising researcher Dr. Robert Jackler testified that one of the founders had thanked him for compiling a database of tobacco ads, saying they were very helpful as they designed Juul’s advertising. Monsees had a very different recollection of the conversation, explaining that they used the archive to learn how not to run a business.
Juul’s empire has always been built on asking forgiveness rather than permission. In 2015, the company launched with its now notorious “Vaporized” campaign, which was called “patently youth-oriented” in a 2019 Stanford white paper authored by Jackler. Colorful ads featured youthful models wearing crop tops and ripped jeans, flirting with the camera as they flaunted their Juuls.
The product’s rollout was accompanied by lavish launch parties, Times Square billboards and an Instagram-heavy social-media blitz. Bowen told TIME–in one of many interviews conducted with company executives over several months–that if he could do it over, the company “would have gone out with a different launch campaign that focused more, as we do now, on the purpose of the product, which is to help smokers switch.” Glantz doesn’t buy that the company didn’t mean to attract youth. Monsees and Bowen consulted him on their device early on, and he says they brushed him off when he said the device would likely appeal to kids. “When they come back and say, ‘This was an accident,’ it’s like, ‘Oh, bullsh-t,'” Glantz says. Bowen says he remembers the meeting but does not recall youth use coming up.
Juul also went to schools and developed classroom curriculums, both ostensibly meant to educate kids about healthy lifestyles and nicotine addiction. But kids who participated in these programs remember them differently. Meredith Berkman’s son Caleb Mintz, now 17, testified before Congress in July that a Juul representative visited his ninth-grade classroom in 2017 and told the students that–though Juul didn’t want them as customers–its product was “totally safe.” Mintz told Congress that his classmates left the meeting more likely to vape, “because now they thought it was just a flavor device that didn’t have any harmful substances in it.” Juul has since halted these programs, but some were conducted as recently as last year. “We had hired educational experts to help us come up with a program that we felt would be helpful to stop kids using Juul,” a company official said in congressional testimony in July. “We then received feedback that it was not well received and in addition, received input from a public-health expert telling us what tobacco companies had previously done, which we were not aware of, and as a result of all of that information we stopped that program.”
For a century, cigarette companies have tried to persuade consumers to switch from one brand to another by making health claims both veiled and blatant. Camel famously bragged in a 1946 ad that “more doctors smoke Camels than any other cigarette.” Around the same time, Lucky Strike claimed it had “removed … the pungent irritants present in cigarettes manufactured the old-fashioned way.”
Juul has adopted that tactic for itself, designing an entire brand based on the idea of “switching” from cigarettes to vapes. Some of its ads seem to crib directly from old cigarette spots, with slogans like “simple, smart, intensely satisfying” and “smoking evolved.” Others apply the old idea of switching to new ground, by calling e-cigarettes a way to “improve the lives of the world’s 1 billion adult smokers by eliminating cigarettes.” Its ads do not explicitly say customers will be healthier if they switch from cigarettes, but “the message is absolutely unmistakable,” Jackler says.
Juul disagrees, saying that switching is not another word for cessation or safer. “They mean very different things,” according to the company. “Switching involves continuing to consume nicotine but from a different device, while cessation is about getting users to eliminate their nicotine consumption altogether.”
The health impact of vaping for adult smokers is one of the most polarizing questions in medicine, and one that scientists say no one can fully answer without years of additional research. Juul, unsurprisingly, is on one end of the spectrum, boasting, as Monsees did at the TIME 100 event in April, that its device represents “one of the greatest opportunities for public health in the history of mankind.” Some experts, like Glantz, are on the other end, arguing that e-cigarettes are “a disaster” and that “the idea that these things are somehow radically safer than cigarettes is just not true.” Many independent researchers say the truth lies somewhere in the middle.
When someone lights a cigarette, tobacco mixes with oxygen, creating an inhalable smoke as well as about 7,000 by-products, around 70 of which are known to cause cancer. E-cigarettes operate under the premise that this combustion, not nicotine, is to blame for most of the health problems associated with smoking, including cancer, heart problems and lung disease. Instead of burning tobacco, Juuls heat a potent liquid cocktail of nicotine salts, flavoring compounds, propylene glycol and glycerine to create an inhalable vapor.
E-cigarettes do contain fewer toxic chemicals, including carcinogens, than cigarettes, so switching could translate to lower rates of smoking-related disease. One 2017 study funded by the National Cancer Institute and the National Institute on Drug Abuse estimated that if almost all U.S. smokers older than 15 switched to vapes, the benefits could save up to 6.6 million lives. “If we look at it from the population perspective, it’s likely that Juul could be lifesaving,” says Andy Tan, an assistant professor in the division of population sciences at Dana-Farber Cancer Institute.
But it’s too simple to look only at “known” carcinogens. It’s not yet clear what impact some of the ingredients unique to e-cigs could have on health, and the products haven’t been around long enough for scientists to know how they affect the body over decades. Studies funded by academic institutions, the National Institutes of Health (NIH), the FDA and the Emphysema Research Fund show links between e-cigarette use and cardiovascular issues, respiratory disease and DNA damage that may be a harbinger of cancer. Using e-cigarettes in conjunction with traditional cigarettes, which the CDC says many users do, may also nullify many of the possible health benefits that come with e-cigs, according to NIH-funded research. And the recent rash of deaths and diseases associated with vaping have made it harder than ever to argue that e-cigarettes are safe.
The cost-benefit analysis is also different for teenagers, many of whom didn’t smoke before they started Juuling and whose developing brains can be harmed by nicotine. These concerns shouldn’t be minimized, says Dr. Michael Siegel, a professor of community health sciences at the Boston University School of Public Health, but he worries that they’ve diverted attention from e-cigs’ public-health potential. Siegel says we risk “regulating [e-cigarettes] out of existence.” The result, he and other advocates fear, could be a world where adult smokers can hardly access a product that could theoretically save their lives, pushing them back toward cigarettes.
Just a few years ago, that concern would have been unimaginable. Juul’s growth benefited from an extraordinary regulatory loophole that will soon slam shut. When Juul launched in 2015, the FDA was still a year away from finalizing its regulatory power over e-cigarettes. That allowed the product to hit consumers’ lungs without ever filing an application with the FDA or having to deal with the strict regulations the agency imposes on traditional cigarettes.
In the same way the federal government has had to play catch-up to regulate tech giants like Facebook and Google, Juul’s technology caught regulators unprepared. Representative Raja Krishnamoorthi, the Illinois Congressman who oversaw July’s hearing and urged the FDA to deem Juul’s health claims illegal, says the FDA also needs to stop the company from subtly marketing its product as a smoking-cessation device. “The FDA has unfortunately been kind of AWOL on this,” he says. “I’m glad to see they’re coming alive right now … better late than never.” The FDA says oversight of e-cigarettes is a “top priority” for the agency. On Sept. 18, Krishnamoorthi sent Juul a letter threatening a subpoena if the company did not produce documents previously requested by Congress.
In May 2020, the FDA will start weighing “the deeply troubling uptake of these products by our nation’s youth against the possible benefits of decreased use of combustible tobacco products by adults,” says Acting Commissioner Dr. Ned Sharpless. If, at that time, e-cig companies cannot prove their products protect public health, the FDA has the right to remove them from the market entirely.
Juul executives have been working for months to keep up with an ever changing regulatory environment, and among the hundreds of open jobs on its website is a dedicated FDA regulatory counsel. There’s no question that the White House’s crackdown on flavors will hit Juul where it hurts. More than 80% of the pods the company sells are flavored, so pulling those from the market will result in a huge revenue hit. But a company source calls the potential result of the government’s actions “short-term pain, but potentially long-term what the category needs,” since driving down youth use is pivotal to securing FDA authorization and keeping Juuls on the market.
Juul has made moves to curb youth use. Last year it deleted its U.S. Instagram and Facebook accounts, which critics argued appealed to teenagers. Juul also limited online sales to those 21 and older, even in states where the legal purchasing age is 18. And a year before the proposed flavor ban, it halted sales of all but mint, menthol and tobacco flavors in stores. The company also emphasizes that it does not sell products in flavors like cotton candy and bubble gum and is discouraging competitors from making such nicotine pods that fit into Juul vaporizers. Most recently, Juul persuaded around 40,000 stores nationwide to implement a point-of-sale system that won’t sell a Juul until it scans a valid ID and that will discourage resellers by rejecting bulk purchases of more than one device and four packs of nicotine pods. The company says it will not do business with retailers that don’t have the system in place within two years.
Still, former FDA Commissioner Dr. Scott Gottlieb says it will be hard for regulators to forget how many kids have become hooked on nicotine because of Juul. “Is it going to be easy for them to get approved? No, it’s not,” he says. “Would I consider taking the pod-based products off the market [if I were still FDA commissioner]? Yes, absolutely I would.”
Until recently, Juul’s Bay Area headquarters had the same vibe as any other Silicon Valley startup. Its hip, open offices on San Francisco’s Pier 70 boast a deck and airy communal work spaces. Framed signs spelling out the company’s values–mission first, think big, deliver quality, debate and commit, go, own it, give back–dot the walls. The 3,800-person workforce, up from about 225 in 2017, includes a healthy share of millennials, and staffers eagerly line up each day when the requisite free lunch is served. “A bigger concern than the FDA is running out of avocados,” CEO Burns joked in July, before the latest developments.
Burns, a former Chobani executive who joined the company in 2017, was intrigued by the challenge of helping a then small company grow as fast as the category it all but created–even if his friends, family and teenage children had misgivings. “It was not a slam dunk,” he says of taking the job. “I have a lot of friends that I’ve known for a long time who kind of look at you and say, ‘Really?'”
Despite the chill startup feel, behind the scenes Juul is just trying to stay above water. In October 2018 it hired publicist Josh Raffel away from crisis-management central: the Trump White House. Raffel is one of many former political staffers at the company, including some from the Trump, Obama and Bush administrations. This year Juul launched a $10 million-plus television ad campaign featuring testimonials from adult users. And, like Big Tobacco giants before it, Juul has begun wooing top researchers to lend the company gravitas. In July, Dr. Mark Rubinstein joined the company as executive medical officer, after years of research at UCSF on adolescent nicotine use. Rubinstein admits the move seems strange, but he says he can better prevent youth use by “working from the inside, [rather] than just writing papers and shouting from the outside.”
Juul also has a strong presence on Capitol Hill, spending $2 million on lobbying so far this year and deploying more than 80 lobbyists working on causes like raising the legal purchasing age for tobacco products to 21. Gottlieb, the former FDA commissioner, told a CNBC reporter in August that Juul and Altria were the “worst offenders,” in terms of going around the FDA to lobby Washington directly. (Juul maintains that it has always supported the need for category-wide federal regulations on e-cigarettes.) This year, instead of targeting only tobacco-friendly Republicans, Juul has started funneling money toward Democrats and supporting groups like the Congressional Black Caucus PAC. That, too, has been a tactic of Big Tobacco, which has long marketed to communities of color.
The company’s most public taste of life in the crosshairs came during the July congressional hearing, when lawmaker after lawmaker questioned Monsees about his company’s role in the youth-vaping epidemic. Monsees demurred on some questions but stayed on message. “[The mission of the company] is to help improve the lives of adult smokers” he said in his opening statement. “We never wanted any non–nicotine user, and certainly nobody under the legal age of purchase, to ever use Juul products.”
Monsees and Bowen are both workaholics who keep grueling travel schedules that often have them launching Juul and accompanying products, like an app that tracks usage, in international markets like the U.K. and Canada. Bowen, the more reserved of the pair, is diplomatic about the constant barrage of criticism their jobs entail, saying it’s “not surprising” and the company “welcomes the concerns, the feedback.” In an interview at Juul’s D.C. office in August, he chooses his words carefully when discussing the balancing act regulators and lawmakers face, but says he’s confident Juul and the FDA will find common ground. “What I would like to see in these discussions is more focus on data [about switching from cigarettes] than just”–he stops, considering the second half of the sentence–“the emotional reaction to these products.”
He never says it outright, but Bowen seems to understand why people react emotionally to e-cigs. A former smoker, he knows that addiction is a sensitive topic. He gets the cynicism over the Altria deal; even he was skeptical at first, he says, and moved forward only once Altria promised prime retail placement and access to its customer database. And he says the company took early critiques over its initial launch campaign to heart. “It was six months and we pulled it,” he says. When pressed on what, aside from marketing, he would have done differently as Juul grew, his tone turns lighthearted–at first. “Now you’re opening up a Pandora’s box,” he says. “It’s too long …”
He trails off, searching for the right words. Finally, he finishes his thought: “You can always do things better, every step of the way.”
More Must-Reads from TIME
- Inside Elon Musk’s War on Washington
- Meet the 2025 Women of the Year
- The Harsh Truth About Disability Inclusion
- Why Do More Young Adults Have Cancer?
- Colman Domingo Leads With Radical Love
- How to Get Better at Doing Things Alone
- Cecily Strong on Goober the Clown
- Column: The Rise of America’s Broligarchy
Write to Jamie Ducharme at jamie.ducharme@time.com and Ang Li at ang.li@time.com