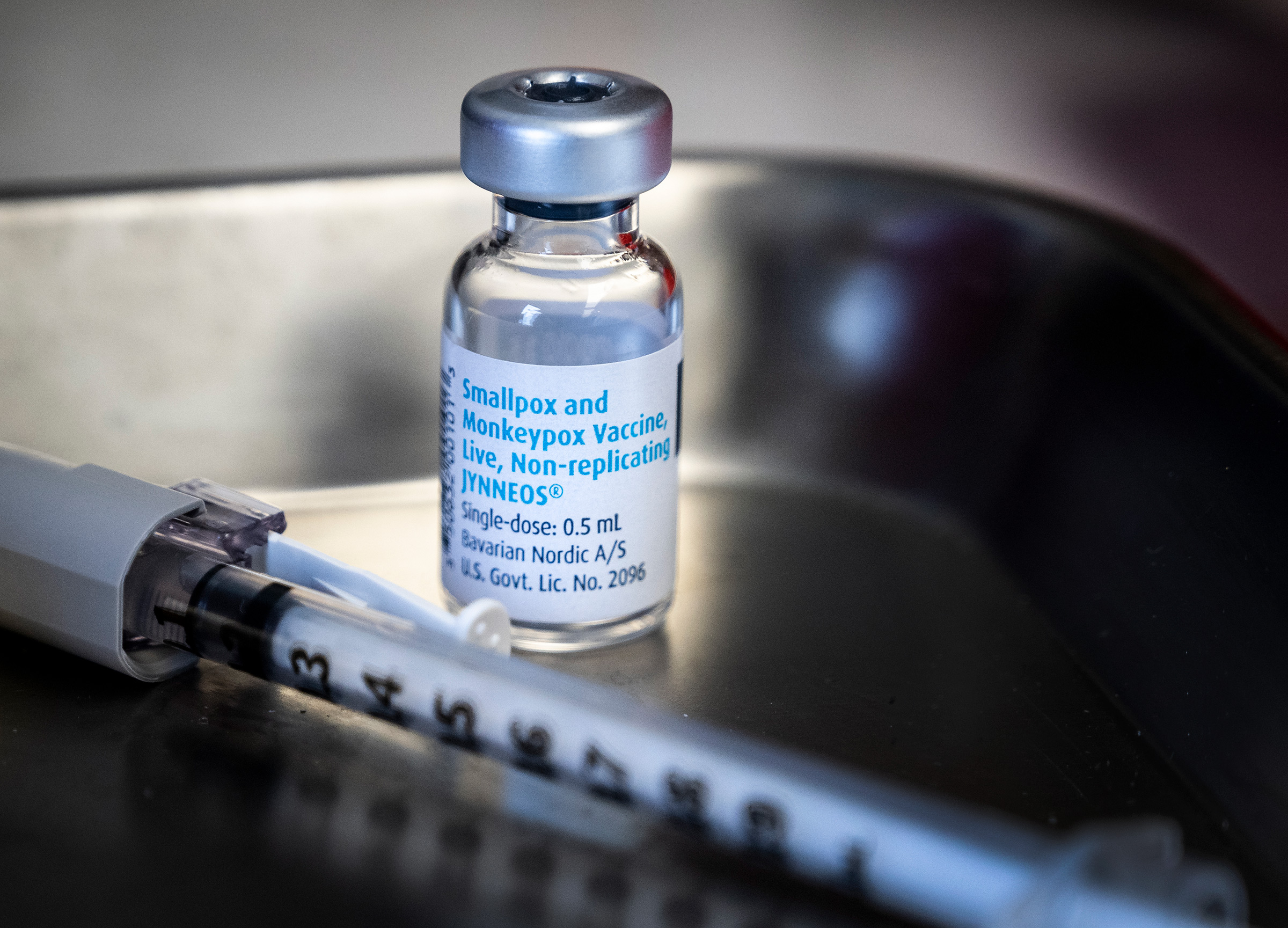
Bavarian Nordic wanted its Jynneos vaccine to be a safer alternative to older smallpox shots, which carry serious side effect risks. Monkeypox wasn’t the vaccine maker’s focus until it applied for U.S. Food and Drug Administration approval in 2018 and regulators suggested licensing Jynneos for use against both viruses, since company scientists had used monkeypox as a proxy for the eradicated smallpox virus. That suggestion proved prescient this year, when monkeypox cases exploded across the globe—and the EU authorized the vaccine’s use for preventing that disease. Though access to Jynneos has been spotty, particularly outside wealthy Western countries, Bavarian Nordic is contracted to deliver more than 10 million doses globally in 2022 and 2023.
- How Donald Trump Won
- The Best Inventions of 2024
- Why Sleep Is the Key to Living Longer
- Robert Zemeckis Just Wants to Move You
- How to Break 8 Toxic Communication Habits
- Nicola Coughlan Bet on Herself—And Won
- Why Vinegar Is So Good for You
- Meet TIME's Newest Class of Next Generation Leaders