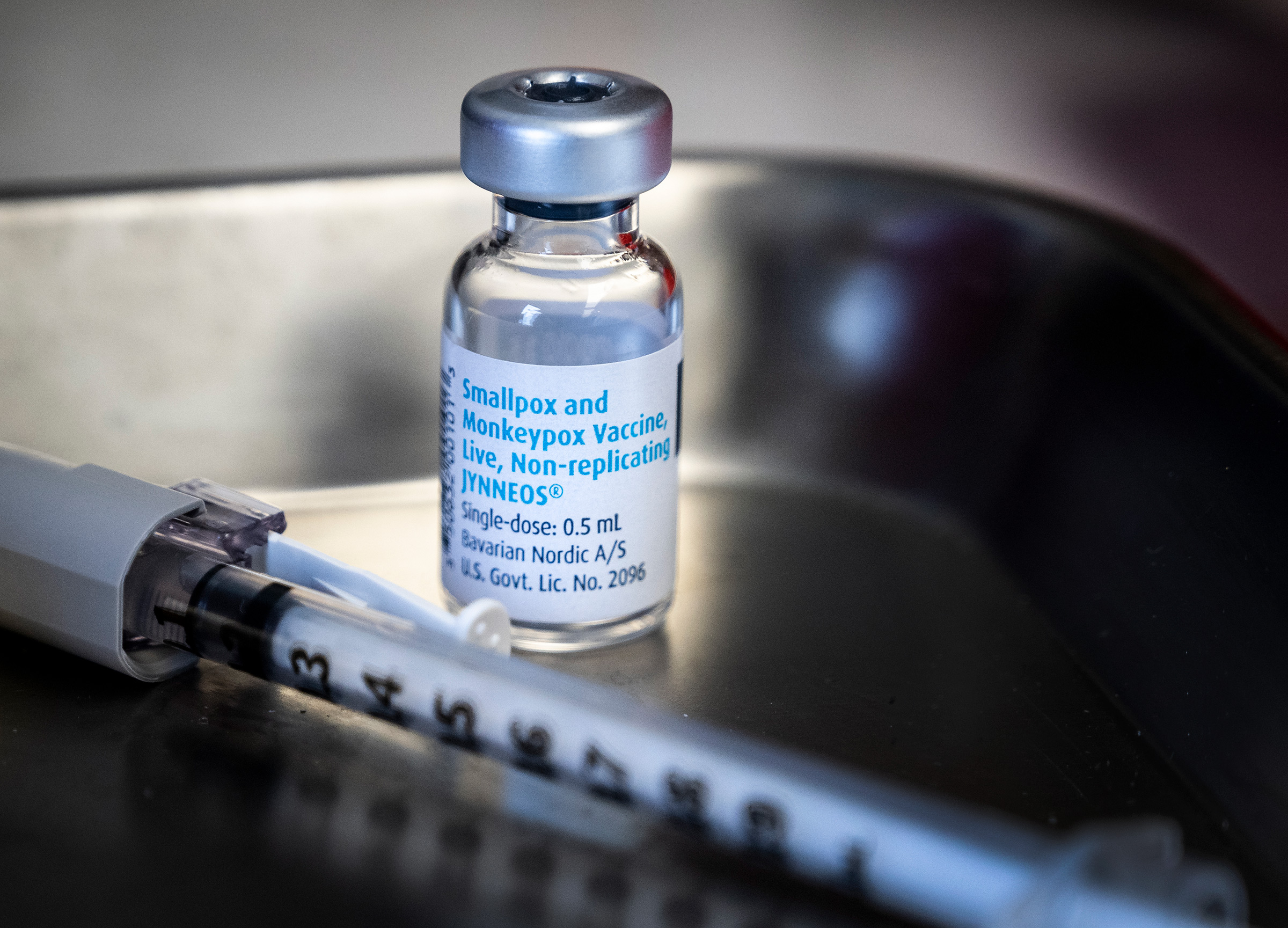
Bavarian Nordic wanted its Jynneos vaccine to be a safer alternative to older smallpox shots, which carry serious side effect risks. Monkeypox wasn’t the vaccine maker’s focus until it applied for U.S. Food and Drug Administration approval in 2018 and regulators suggested licensing Jynneos for use against both viruses, since company scientists had used monkeypox as a proxy for the eradicated smallpox virus. That suggestion proved prescient this year, when monkeypox cases exploded across the globe—and the EU authorized the vaccine’s use for preventing that disease. Though access to Jynneos has been spotty, particularly outside wealthy Western countries, Bavarian Nordic is contracted to deliver more than 10 million doses globally in 2022 and 2023.
- Donald Trump Is TIME's 2024 Person of the Year
- Why We Chose Trump as Person of the Year
- Is Intermittent Fasting Good or Bad for You?
- The 100 Must-Read Books of 2024
- The 20 Best Christmas TV Episodes
- Column: If Optimism Feels Ridiculous Now, Try Hope
- The Future of Climate Action Is Trade Policy
- Merle Bombardieri Is Helping People Make the Baby Decision