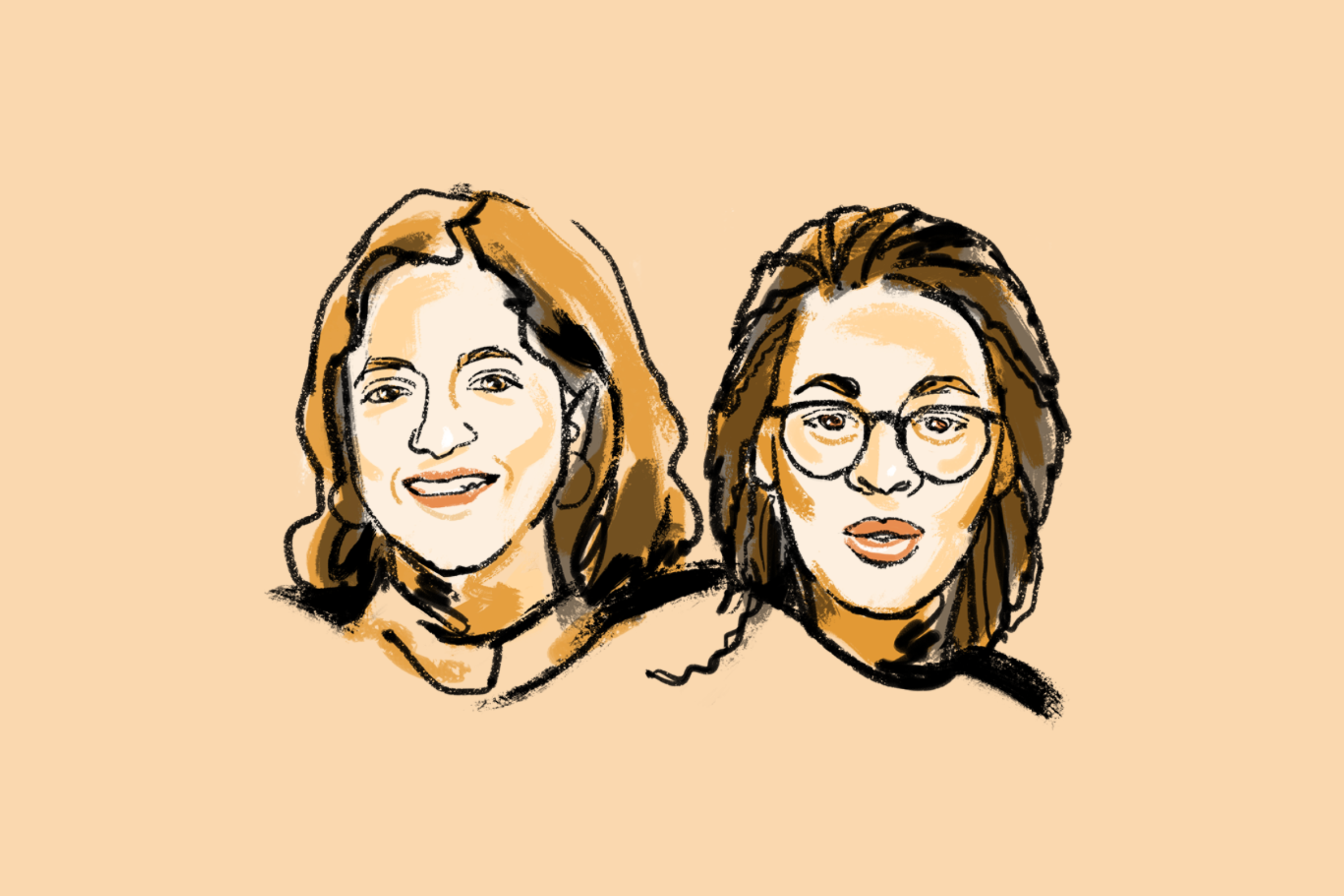
Tuberculosis survivors Nandita Venkatesan and Phumeza Tisile both lost their hearing during their bouts with a multidrug-resistant version of the disease—a side effect of the toxic cocktail of drugs they took during treatment. Johnson & Johnson has created a safer and more effective drug to treat TB, but patent laws made it inaccessible for many worldwide—and, with the initial patent set to expire in many countries this past July, the company filed for another, which would extend its monopoly.
Together with Médecins Sans Frontières, Venkatesan and Tisile, longtime advocates based in India and South Africa respectively, filed a petition with the Indian government to deny the secondary patent—thus making way for cheaper generics. In March, India rejected the secondary patent, a landmark victory that will help make the drug available at a much lower price. Separately, Johnson & Johnson announced an agreement this summer that will make generic versions more accessible in lower-income countries. “We had to undergo what we had to undergo,” says Venkatesan. “But maybe we could prevent this from happening to others.”
Correction, Sept. 13
The original version of this story misspelled one of the advocate’s names. Her name is spelled Phumeza, not Phuzema.
- How Donald Trump Won
- The Best Inventions of 2024
- Why Sleep Is the Key to Living Longer
- Robert Zemeckis Just Wants to Move You
- How to Break 8 Toxic Communication Habits
- Nicola Coughlan Bet on Herself—And Won
- Why Vinegar Is So Good for You
- Meet TIME's Newest Class of Next Generation Leaders