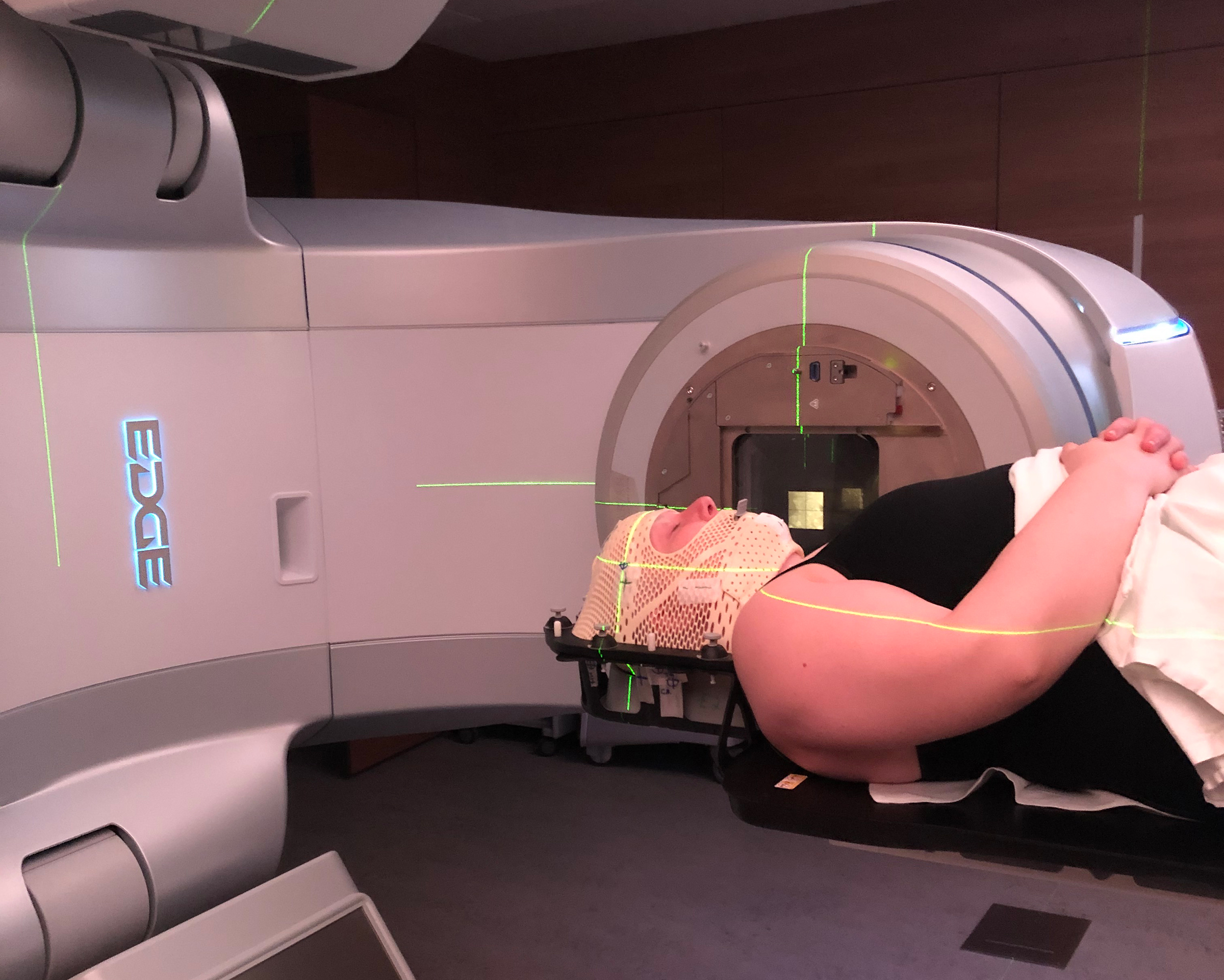
Tori Geib was already on high alert when COVID-19 hit last winter. Diagnosed with metastatic breast cancer in 2016, the Ohio chef went from one chemotherapy regimen to another in an effort to outrun the cancer that had spread from her breast to her bones, lungs and liver. To protect her-self from infections, even before the pandemic she often wore masks when she went out in public and carried hand sanitizer at all times. But COVID-19 presented a new and daunting challenge.
At some point, Geib knew, she would exhaust all approved treatment options and would need to move to experimental therapies. But when COVID-19 began to burden hospitals, many suspended clinical trials. “It made what limited options I had even more limited,” she says. “When your cancer is growing and progressing, you want to know what the next thing is that you will have access to. COVID-19 brought in a new fear: Will that research or trial be there when I need it?”
Three months ago, Geib learned her cancer had progressed, so she again changed to a different chemotherapy. At the end of June, she learned the cancer had spread to her brain, so she received radiation treatment. She is also taking another off-label therapy while waiting for more clinical trials to become available near where she lives. “I’m trying to navigate the system and find the next thing I need to go to.”
Estimates of how many cancer patients enroll in clinical trials range from 2% to 8%. But since the pandemic began, the National Cancer Institute (NCI), which sponsors many cancer trials in the U.S., says enrollment in trials has dropped by about 10% each month. The potential impact is profound. “First, it’s a missed opportunity for patients to actually avail themselves of participating in a clinical trial if the trial is on hold or temporarily suspended or even closed,” says Dr. Richard Schilsky, chief medical officer and executive vice president of the American Society of Clinical Oncology. “The longer[-term] impact is that the time to complete trials is going to be longer than originally planned because enrollment has taken a big dip for a period of months, and it will take time to make that up. That means it will take longer to get an answer to a trial and longer to potentially bring new therapies to patients.”
With limited resources, many study sites decided to triage their clinical trials, suspending early-phase studies, in which the benefit of the experimental treatment is largely unknown, in favor of keeping later-stage studies, which test treatments that have already shown some promise. “All of these decisions are based on benefit-risk assessment,” says Schilsky. “What is the risk of interrupting, delaying or discontinuing a patient on a cancer treatment, especially if they have an aggressive, rapidly progressive cancer, vs. the risk of continuing treatments that require them to come into a health care facility for frequent visits, risking exposure to COVID-19 infection?”
Normally, study conditions require patients to come to the trial site to receive their medications along with instructions on how to take them. To keep some trials going during the pandemic, the Food and Drug Administration and the NCI worked to allow study sponsors to ship experimental therapies directly to patients. Similarly, virtual checkups replaced in-person visits when possible, further reducing COVID-19 risk for trial participants.
Time will tell how those changes affect the results of clinical trials; for example, it’s possible the lack of medical oversight will affect compliance with taking medications. But Schilsky notes there may be a silver lining. “Many of the adaptations make it easier for patients to participate in clinical trials,” he says. “So if they work, there may be no reason to go back to the old way of doing things. Hopefully the adaptations made during the pandemic will position us to do clinical trials more effectively than they’ve been done in the past and ultimately open them up to more patients.”
More Must-Reads from TIME
- Cybersecurity Experts Are Sounding the Alarm on DOGE
- Meet the 2025 Women of the Year
- The Harsh Truth About Disability Inclusion
- Why Do More Young Adults Have Cancer?
- Colman Domingo Leads With Radical Love
- How to Get Better at Doing Things Alone
- Michelle Zauner Stares Down the Darkness
Contact us at letters@time.com