On the evening of Nov. 7, Steffanie Strathdee sent out a cryptic tweet: “#Phage researchers! I am working with a team to get Burkholderia cepacia phages to treat a 25 y old woman with CF whose infection has failed all #antibiotics. We need lytic non-lysogenic phage URGENTLY to find suitable phage matches. Email if you can help!” The message was retweeted nearly 400 times.
To the average social-media user, the tweet might as well have been written in another language, but to those who know Strathdee, it was a rallying cry. Strathdee is the associate dean of global health science at the University of California, San Diego (UCSD), and she’s part of a small but growing community of scientists advocating for an experimental treatment for superbug infections. The treatment, called phage therapy, uses bacteriophages, which are tiny viruses that appear to have an uncanny ability to destroy some of the most lethal strains of drug-resistant bacteria. The treatment is not without controversy, however.
The young woman Strathdee was trying to help was Mallory Smith, a 25-year-old in critical condition at the University of Pittsburgh Medical Center. Over a decade earlier, possibly during one of her many hospital stints as a cystic-fibrosis patient, Smith had acquired a drug-resistant Burkholderia cepacia infection. For a while, her doctors thought they could control it with antibiotics, but the bacteria kept fighting back, growing ever stronger in her lungs. They went to extreme measures to rid her of the infection, even performing a double lung transplant, but the bacteria had also migrated to her sinuses. After her surgeries, the bug came back in full force.
Desperate to save his daughter, Mark Smith went looking for an alternative. He stumbled on a news article about how an infectious-disease expert named Steffanie Strathdee almost lost her husband Tom Patterson to a superbug infection. At Strathdee’s urging, her husband’s doctors tried to cure him with an experimental treatment in which viruses are used to target and kill drug-resistant bacteria. It sounded almost too simple to be plausible. But ultimately, phage saved his life.
After reading about this remarkable turnaround, Mark sent Strathdee an email, prompting her viral tweet. Within a week, a lab in Maryland confirmed it had identified a phage virus that might be able to kill the bacteria. The scientists packed up the phages and sent them to Pittsburgh. They arrived at the hospital on Nov. 14 at about 6 p.m.
“I want phage to work,” said Mallory’s mother Diane Shader Smith from her daughter’s bedside in early November. “But even if it doesn’t, we need to shine the light on this treatment.”
Every year in the U.S., at least 2 million Americans are diagnosed with an infection that doesn’t respond to antibiotics, and according to conservative estimates, 23,000 of them will die from their infections. The U.S. Centers for Disease Control and Prevention acknowledges that these figures are likely an underestimate: hospitals are not required by the federal government to report the number of superbugs they encounter, and death certificates rarely mention them directly (instead, complications from other ailments are often cited). Experts caution that the day is not far off when a paper cut or an ear infection turns lethal. Economists predict that by 2050, as many as 10 million people per year will die from drug-resistant infections.
“This is a crisis,” says Ry Young, director of the Center for Phage Technology at Texas A&M University, which studies phage treatments for superbugs. “People are completely unaware of the danger we are in.”
Indeed, the scope of the threat posed by superbugs remains poorly understood by the general population, despite the fact that the World Health Organization calls superbugs an imminent threat to human health. What’s more, there isn’t much hope on the horizon when it comes to new drugs. “There are no new antibiotics coming, and if they do come, bacteria tend to develop resistance to the drugs within a year,” says Young.
The paucity of treatment options is fueling interest in phage, which some researchers are calling a critical weapon against the kind of infections that threatened the lives of Mallory Smith and Tom Patterson. For now, however, phage is not widely available in the U.S. Only a handful of people have received it, and it requires special clearance, because it isn’t yet approved by the Food and Drug Administration (FDA). Before phage can go mainstream, the science needs to catch up–which experts are hopeful will happen in 2018, when a number of trials will kick off in the U.S. If the science bears out phage’s promise, it would be life altering–and life saving–for the millions of people around the world who contract superbug infections every year.

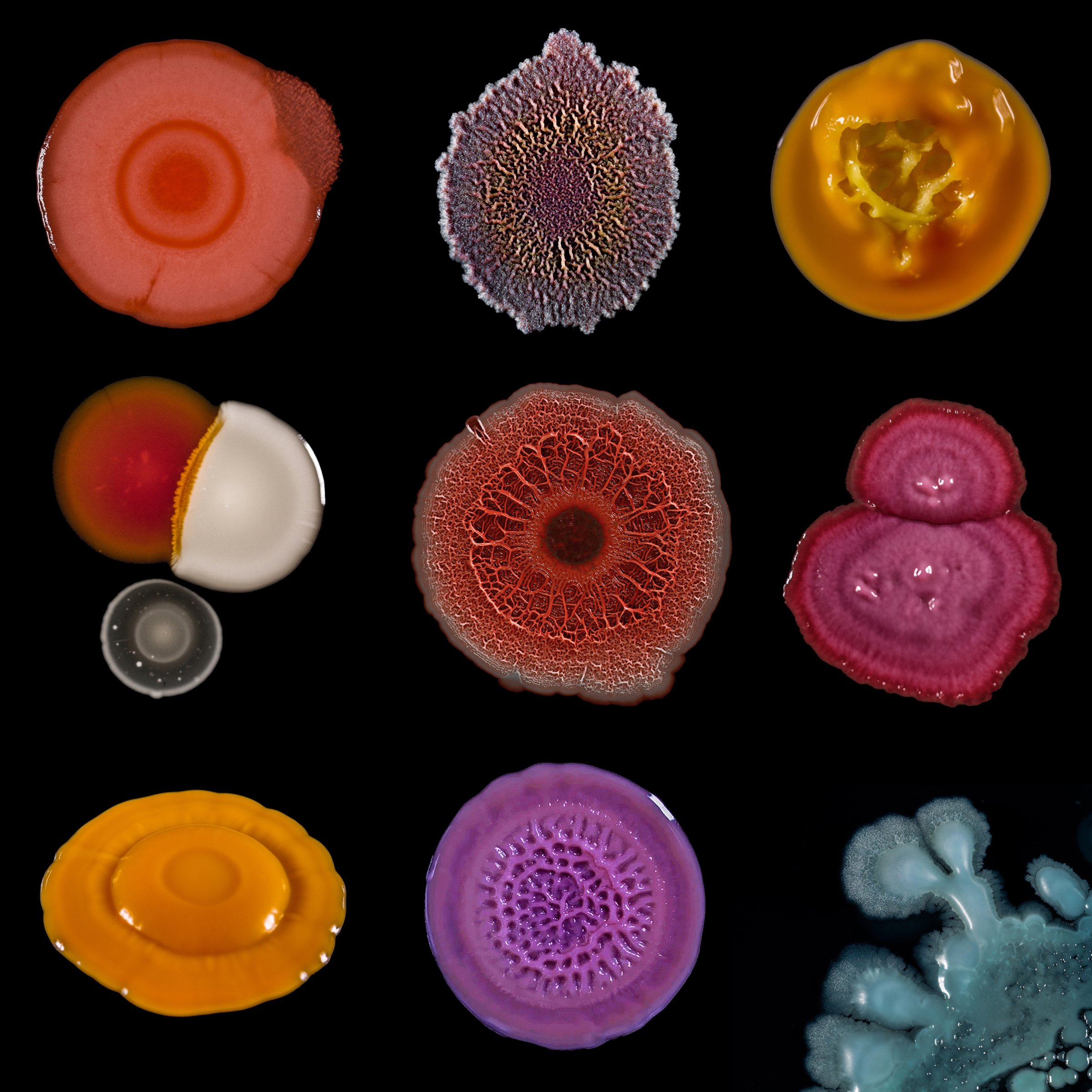
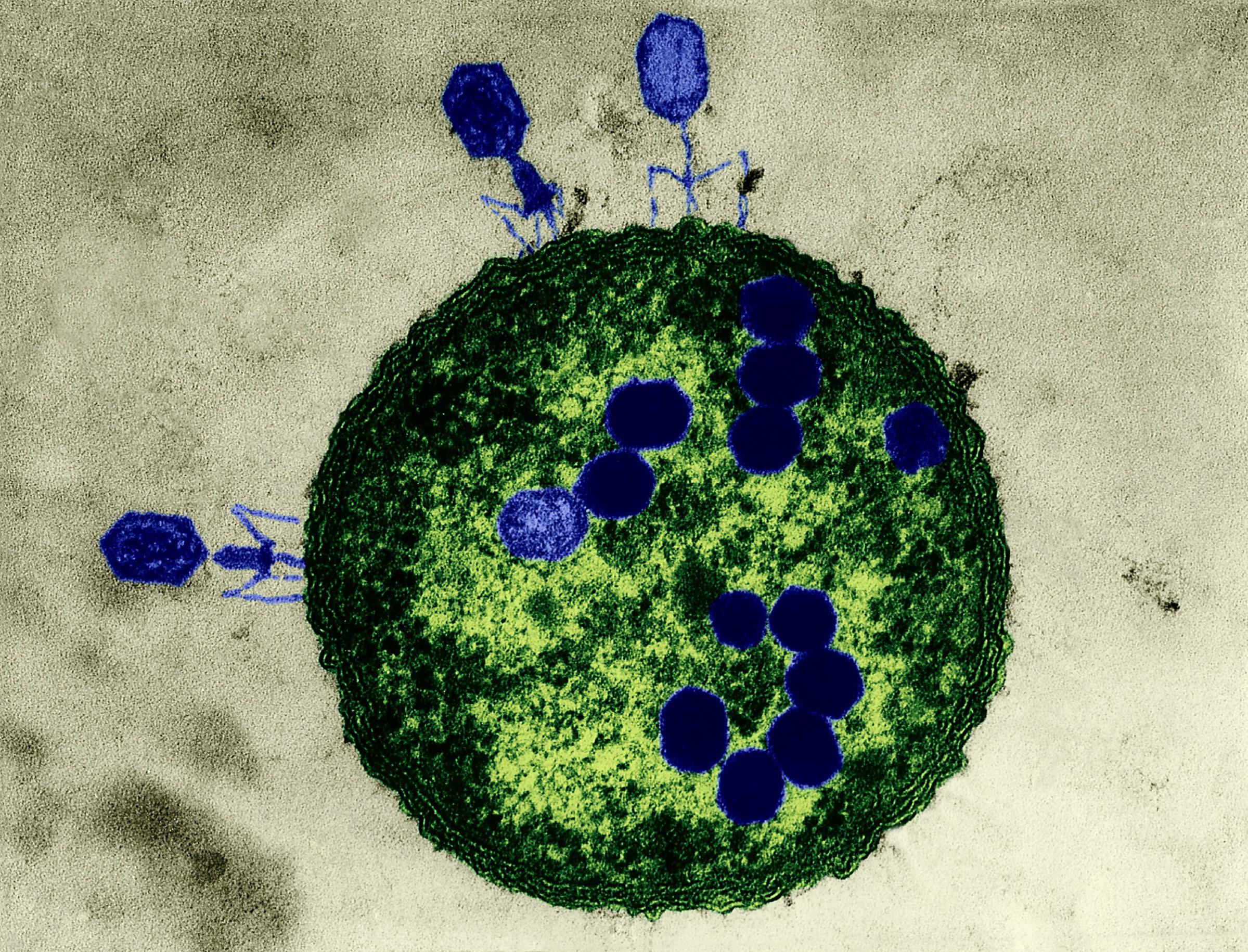
Phages are the most ubiquitous bacteria fighters on the planet. Scientists estimate that there are over 10 million trillion trillion phages, which is more than any other organism in the world. Phages work by injecting their DNA into bacteria cells, where they rapidly replicate, causing bacteria to burst open and die. What’s special about phages is that for the most part, each strain attacks only a specific kind of bacteria that they’ve evolved to kill, which means phages provide a more personalized approach than, say, broad-spectrum antibiotics, which target a wide range of bacteria–even the good kind. Despite the elegance of the treatment, the Western medical community had all but abandoned it until very recently.
Discovered by Frederick Twort in England in 1915 and Félix d’Hérelle in France in 1917 (there’s debate about who was truly first), bacteriophages were used in the U.S. and around the world to treat infections throughout the 1920s and ’30s.
Even back then, however, the treatment was divisive in the medical world. “From the very discovery of phage, this field has seen personal attacks, disputes of priority, massive egos and international politics,” wrote Dr. William Summers, a medical historian at Yale University, in a 2012 paper on phage. “All of these are part of the rocky history of phage therapy.”
When mass production of antibiotics took off in the 1940s and ’50s, doctors moved away from phage and embraced the drugs. Today more than 266 million antibiotic prescriptions are written in the U.S. per year.
In the last century, scientists in Eastern Europe continued to use phage, but in the U.S. it fell out of vogue, developing a reputation as being an unsafe and clunky treatment. Modern phage researchers say that’s because when phage therapy was first used in the U.S. 100 years ago, other aspects of science were too poorly understood. (This was, after all, before the discovery of DNA.) Still, the reputation stuck. Today people who want to try phage therapy are required to get what’s called an “emergency investigational new drug” application from the FDA indicating that they are out of other treatment options and are very sick. That’s how Strathdee’s husband Tom Patterson was able to try it.
In late 2015, Strathdee and Patterson, a professor of psychiatry at UCSD, were celebrating Thanksgiving in Egypt when Patterson became violently ill. Diagnosed with pancreatitis, Patterson was flown for treatment to Frankfurt, where physicians discovered that he was infected with a deadly superbug called Acinetobacter baumannii. Patterson was given potent antibiotics and eventually got the green light to travel back home to San Diego.
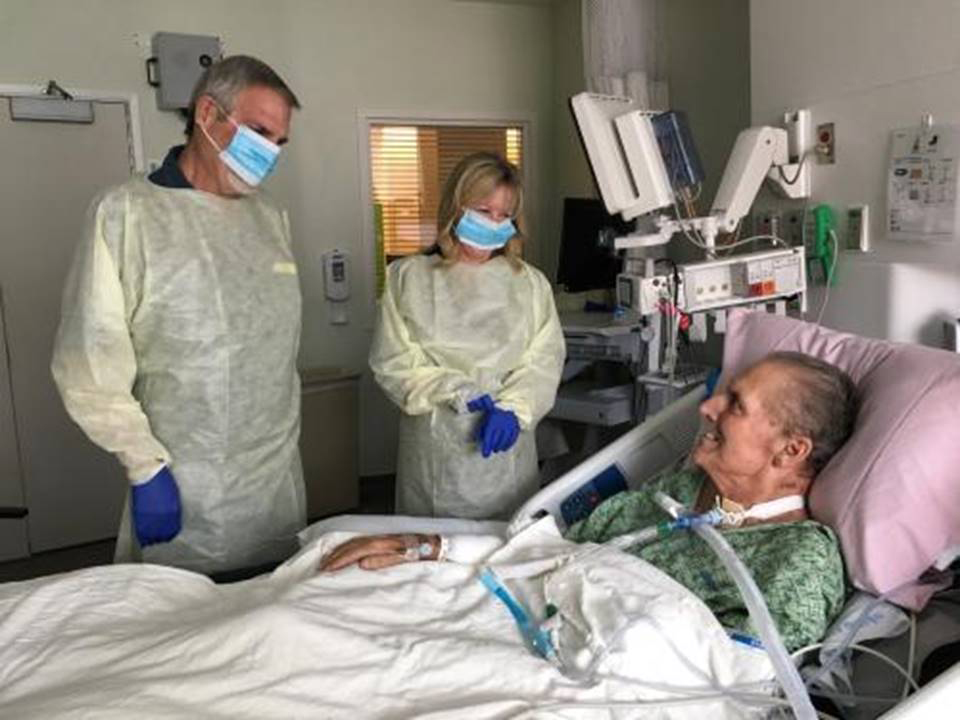
Patterson was put under the care of several doctors, including his friend Dr. Robert “Chip” Schooley, a professor of medicine in the division of infectious diseases at UCSD. “Tom was as sick as he could be,” says Schooley. “He was either going to die or we were going to come up with something very different.”
As an infectious-disease expert, Strathdee understood the threat of antibiotic-resistant infections all too well. She felt helpless watching her husband die, so she started researching alternatives. A colleague mentioned that a friend of hers had once traveled to Tbilisi, Georgia, to receive something called “phage therapy” and came back “cured.”
Strathdee approached Schooley about giving phage a shot. Although he wasn’t convinced it would work, he agreed. With an emergency approval from the FDA, Patterson was given a phage cocktail on March 15, 2016, and another two days later. Three days after that, he woke up from his coma.
“I am still weaker than I was before,” says Patterson, who is back at work. “But I feel I have a new life entirely, and it’s wonderful to have that gift.”
Since Patterson’s success story was announced to the public in April 2017, nearly all the physicians involved in his care receive weekly emails from people whose loved ones are suffering from superbug infections. (Strathdee also landed a deal for a book on the subject. It’s due in 2019.) Experts are hopeful that before too long, phage will clear FDA approval, making it more widely available to the people who need it most.
Phage therapy has some unique benefits over antibiotics. For one, bacteriophages can be found everywhere on earth, even in sewage. Second, it attacks only the targeted bacteria, not the so-called good bugs in the host. Third, it can be done quickly: scientists can create a phage cocktail and provide it to patients within 48 hours of a superbug-infection diagnosis in some cases. And even though bacteria can become resistant to phage, there are an infinite number of strains of the viruses–not so with antibiotics. During Patterson’s treatment, the bacteria grew resistant to his initial phages, but the doctors were able to tweak the treatment with new strains until he cleared the infection.
Proponents of phage research say the treatment will never replace antibiotics altogether–for routine infections, the drugs will always be more convenient and easier to use. But if phage is approved by the FDA, it could become a powerful antidote to increasingly common superbug infections.
For that to happen, researchers need to perform clinical trials that produce a better understanding of the basic mechanisms of phage therapy so that successes in one person can be replicated in other people at scale.
In 2018, two small biotech companies in the U.S.–AmpliPhi Biosciences and Adaptive Phage Therapeutics (APT)–will launch clinical trials that will attempt to answer some of the key questions about phage.
“In the future, if we can cut the time down, phage might be better than antibiotics,” says APT founder Dr. Carl Merril.
Merril, a former National Institutes of Health scientist, came out of retirement at the age of 80 to launch APT in October 2016. Merril has long been an advocate for using phage–he was featured in a 1971 issue of TIME for his work on the viruses–but his research never made it beyond the lab and into human testing.
Merril says he had long since given up on the possibility that phage therapy would ever become a mainstream treatment. That is, until Strathdee called him for advice about how to save her husband’s life.
“This is sort of like a dream,” says Merril, who advised doctors on how to administer phage to Patterson. It was Patterson’s stunning recovery that inspired Merril to start APT.
“I am very old, and I remember what it was like before penicillin,” says Merril. “I lost a lot of my classmates. It could happen again. If we don’t deal with antibiotic resistance in real time, it will get worse.”
APT is working on perfecting ways to scan bacteria samples against a massive library of bacteriophages that was collected by the U.S. Navy. Using proprietary algorithms that rely on artificial intelligence, APT then hopes to be able to quickly match bacterial strains with the phages that might be able to thwart them.
Merril envisions a time when APT can provide pharmacies with kiosks of phage strains, which APT has already trademarked as PhageBanks. If his dream comes true, then within one hour, infection samples would be screened and matched with the appropriate phages, which could be dispensed from the kiosks in single-dose vials. APT estimates that there’s a $60 billion market opportunity for phage-based treatments.
Researchers caution that before that happens–before phage is embraced as a standard treatment as opposed to an experimental last resort–there’s a lot of work to be done.
“It’s unsustainable how we are doing it now,” says Texas A&M’s Young, who worked on both Tom Patterson’s and Mallory Smith’s cases. “Every time we try phage on a new patient, it’s automatically a crisis, because you have someone who is dying. Everyone wants to help, but we need a system.”
Young says he worries that if mistakes are made in the rush to get phage to patients, the field could end up in “we told you so” territory. “People should understand that an old therapy is back,” he says. “But it’s not yet commercially exploitable.”
Since treating Patterson in 2016, the medical team at UCSD has treated three other people using phage, and two appear to be doing well.
“Thank God for Steffanie,” says Josephine Willson, whose husband John was treated with phage at UCSD in May after contracting a resistant Pseudomonas infection. “If she didn’t fight for her husband, I wouldn’t have mine here today.”
Josephine says her experience with her husband’s infection, which he’d been fighting for over six months before receiving phage therapy, has woken her up to the ever growing threat of superbugs. “All I knew was that overuse of antibiotics could be detrimental at some point in time,” she says. “I did not know we are already at a point where antibiotics cannot kill a bacteria you have. I did not know that.”
Schooley, who now travels across the U.S. educating medical groups about phage, says tackling the superbug problem will require more education as well as better use of antibiotics and new therapies. “We need to use antibiotics intelligently,” says Schooley. “But even if we stop using every antibiotic there is, there will still be multidrug-resistant organisms. We need to minimize their ongoing evolution by using antibiotics appropriately in the hospital and in agriculture–and we also need to think about novel approaches to head off these superbugs.”
Many scientists have renewed hope that phage will be one of those solutions. “An international community has been galvanized,” says Strathdee. “We need to keep up the momentum.”
Mallory Smith’s parents also want to help phage research continue so that it may one day help other people–even though it couldn’t save their daughter.
By the time the phages made their way to Mallory in Pittsburgh, it was too late. She started the therapy but died the next day.
“We were two days short,” Strathdee says. “Mallory’s case symbolizes so much.”
More Must-Reads from TIME
- Why Trump’s Message Worked on Latino Men
- What Trump’s Win Could Mean for Housing
- The 100 Must-Read Books of 2024
- Sleep Doctors Share the 1 Tip That’s Changed Their Lives
- Column: Let’s Bring Back Romance
- What It’s Like to Have Long COVID As a Kid
- FX’s Say Nothing Is the Must-Watch Political Thriller of 2024
- Merle Bombardieri Is Helping People Make the Baby Decision
Contact us at letters@time.com