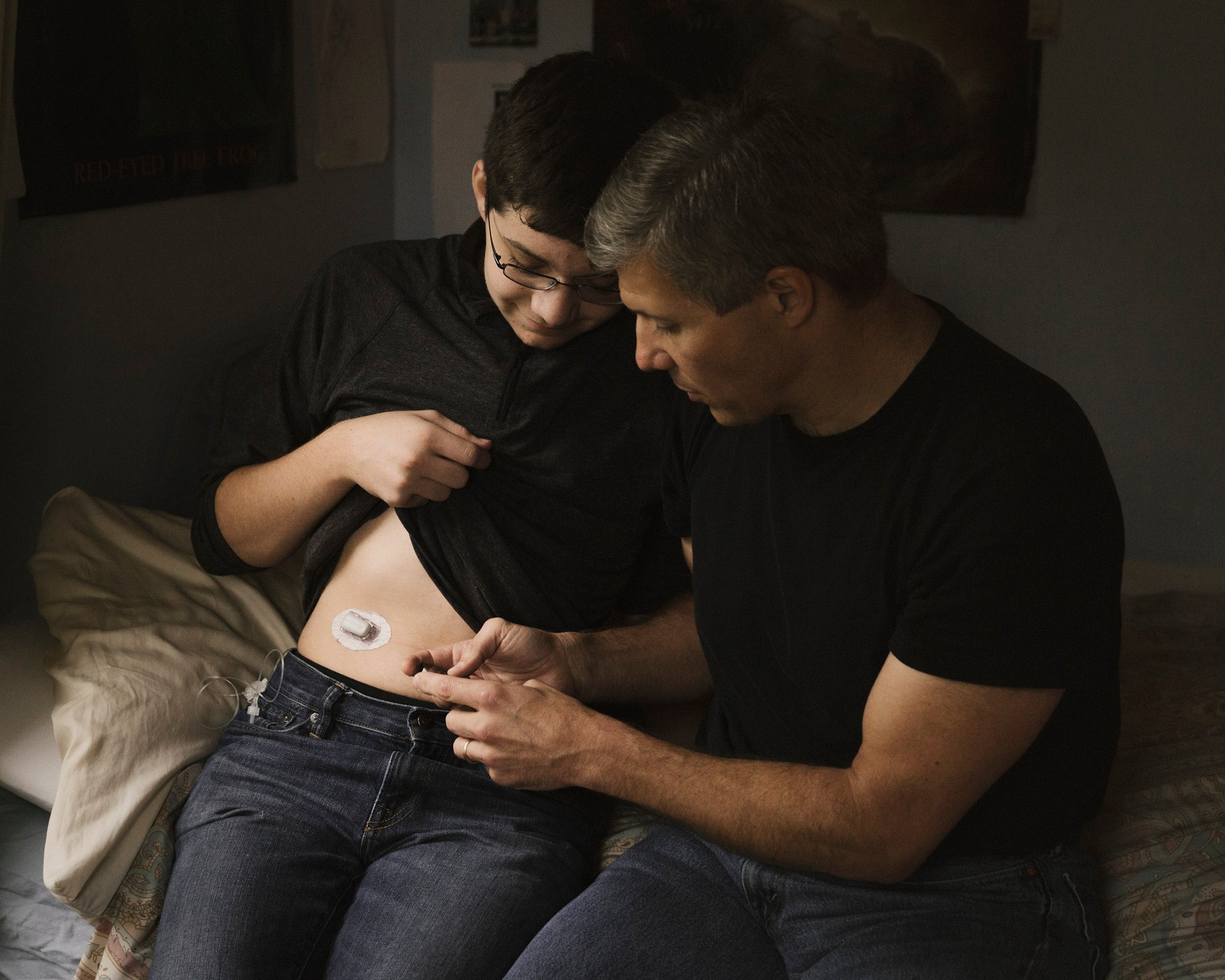
Late Last Summer, 15-year-old David Damiano spent more than 24 hours away from his parents for the first time in his life. He went to summer camp, but it was hardly the same experience most kids get to enjoy. David, like 1.25 million other Americans, has Type 1 diabetes, which means that his life depends on constantly tracking and precisely adjusting his blood sugar. If it’s too high, he feels nauseated and has to inject himself with insulin through a pump attached to his body. If it’s too low, he becomes delirious and shaky and needs to eat something high in carbohydrates–fast.
Even when he’s not feeling symptoms, he has to continually tweak his insulin levels up or down because if they aren’t stable, he’s at risk for an emergency-room visit and long-term consequences ranging from blindness to kidney failure to amputations. All of us require the same nonstop insulin adjustments. But for most of us, the job is done automatically by a pancreas that works properly. David doesn’t have one of those.
On the first day of camp, David went on a two-mile (3.2 km) hike with his campmates and forgot to bring his usual bag of snacks. His blood sugar plummeted, and he was nowhere near food. “I tend to forget about my diabetes most often when I’m relaxed and just feeling like a normal kid,” he says. A few nights later, he awoke feeling angry–the disease can toy with your hormones–and sick to his stomach. His blood sugar was high, and he realized his insulin pump wasn’t working. After frantically calling his dad at 1:30 a.m., David fitted himself with a backup pump. “I definitely could do camp again, but I’m not sure I’m willing,” he says. “It’s just hard.”
What David and other diabetics need is something that automates the moment-to-moment monitoring and medicating that can suck so much of the joy out of life. Any child’s parents would wish for such a solution–but David’s are in a position to help design it.
His mother Dr. Toby Milgrome is a pediatrician who diagnosed her son’s Type 1 diabetes when he was 11 months old. His father Ed Damiano, a professor of biomedical engineering at Boston University, has made it his mission to build a portable, wearable bionic pancreas–a device he hopes to have on the market as early as 2017, the year David is set to go off to college.
For decades, the promise has been that a diabetes cure is just five years away–a projected target that has never come any closer. A bionic pancreas is not the same as a cure, any more than an artificial heart is a cure for cardiovascular disease. But for many people with diabetes, it could prove to be the next best thing. Any device that systematically changes what it means to live with a chronic disease is rare. A device that did that for diabetes could be a life changer for people with the disease. It could also translate into profits for Damiano–Type 1 diabetes accounts for $5 billion in health care costs each year–which is why a number of other research groups are working on their own versions of the bionic pancreas. (For now, Damiano is focused on Type 1–the kind of diabetes that cannot be prevented and usually strikes people when they are children–as opposed to Type 2, which is more common and often caused by lifestyle and diet.)
Damiano is convinced he can bring his bionic pancreas to market in 2017. Already about 260 people with diabetes have tried a form of the device in clinical trials–and the experience has been transformative, some say. At the end of one recent trial, an 11-year-old boy liked the bionic pancreas so much that he ran away from the investigators conducting the test, and it took them over an hour to get the device back.
Matt DiPadua, 35, a hospital worker who tried the device for 22 days, described the time he spent with it as “bliss,” the kind that almost made him sorry he’d participated in the trial at all because giving it back was so difficult. “I felt like I was 15 again,” he said. “I’m so depressed getting it off. I would trade anything to be normal again.” For DiPadua and millions of others, normal may be closer than it’s ever been before.
A CHANGED LIFE
Diabetes tends not to arrive quietly, and David’s case was no exception. When he was 11 months old, his mother noticed that in the span of just one week, her once vivacious son, who was teaching himself to walk, became lethargic and seemingly indifferent to his surroundings–often staring into space. He lost weight, had an insatiable thirst and burst his diaper from urinating so much. At the end of the week, Milgrome took him into her office for lab work. “I knew it was nothing simple at that point,” she recalls. “I had brain tumor, leukemia and diabetes on my mind, and I also had a hefty dose of denial, that it was nothing.”
David’s blood sugar was 800 mg/dl–normal is 70 to 120 mg/dl–and a diagnosis of diabetes was confirmed. Milgrome rushed David to the hospital and spent the night curled up with him. The worst-case scenario the family faced was that the boy would be dead in days; the best scenario was that he would survive–but live a radically different life than they had expected.
Just how radically is something only people with diabetes and their families fully appreciate. In the years ahead, David’s goldfish crackers were counted, the noodles he ate were measured in a quarter-cup, and his parents went with him on every school trip. The vast majority of people with diabetes monitor their blood sugar by pricking themselves with a needle 10 or more times a day and squeezing a drop of blood onto a sensor strip. If they need insulin, they must slip away somewhere private and inject themselves with a syringe. “I carry around candy bars. I have holes in my fingers from checking myself,” says DiPadua.
There are easier ways to go about things. Some diabetics, like David, use an insulin pump and a continuous glucose monitor (CGM), both of which are attached to the body on the lower stomach–worn like a sort of life-giving holster. The CGM checks blood sugar every five minutes and beeps an alert if levels are high or low. The pump must then be operated manually.
Damiano has modified his son’s system, devising a way to hack David’s CGM so it uploads to the cloud and Ed can constantly read the numbers. Still, about 20 times a day, David’s monitor issues a loud beep, and no matter where he is, he must adjust his insulin dose.
CGMs and pumps are certainly an improvement over the needle-and-syringe protocol, but only 25% of people with diabetes opt for that higher-tech route. Insurance typically covers the insulin pump, but not always. A pump costs about $6,500 on its own and has separate costs for pieces like insulin cartridges and reservoirs, all of which add up to about $1,500 a year out of pocket. A CGM costs $500 to $1,000 for the primary device, and it’s about $50 to $100 every week for the replaceable sensor needle that sits under the skin. CGMs are not covered by Medicare nor, in most states, Medicaid.
GETTING TO WORK
Within months of David’s 2000 diagnosis, Damiano decided it was time to change the game. At the time, he oversaw a staff of scientists and graduate students at the University of Illinois at Urbana-Champaign (he later moved to Boston University), studying the mathematical models of bodily systems, like the flow of fluids in the ear involved in balance. But the bionic-pancreas idea had been teasing at him ever since David got sick. He put one of his graduate students, Firas El-Khatib, on the task to help him develop an algorithm for the accurate delivery of insulin and a second hormone called glucagon. With funding from diabetes-research foundations, they had a working model for experiments on pigs in late 2005.
The various generations of devices that grew from that and have been tested in humans are surprisingly compact–about the size of an iPhone. Blood-sugar levels are monitored using a typical CGM system that relies on probes inserted into the skin. Readings are taken every five minutes, and then, depending on blood-sugar levels, a tiny pump releases insulin to bring the sugar down or glucagon to bring it back up, thereby keeping the blood sugar steady.
The prototype in the trials is not sexy–the components are cobbled together–but it works, and everything can be monitored with an iPhone app. The goal is to allow diabetics to go about their day without having to make a single decision about their care.
The 110 people who have tried the most recent version of Damiano’s device have participated in one of the four clinical trials he has conducted–each bigger and more ambitious than the one before it. The bionic pancreas has successfully worked in people ages 6 to 76 and weighing 47 lb. to 283 lb. (21 kg to 128 kg). The longest anyone has worn it is about 22 days.
Results from the last published study, in the New England Journal of Medicine, show that 81% of people on the bionic pancreas had better blood-sugar control than with their standard treatment. For others, the bionic pancreas did not lead to better blood-sugar control than their regular treatment. Some also felt nauseated. Currently, four institutions are participating in a trial of 40 adults who are allowed to go about their normal routines without the in-person supervision that had been required earlier.
THE FINAL PUSH
Damiano plans to start the final, pivotal trial in 2016, one that will last several months and include hundreds of participants. That study will involve a far more elegant, far more portable unit than the current prototype. All of the hardware will be packed into a single unit that will be palm-size or smaller and will operate under a new, better algorithm Damiano and his team are writing for an upgraded operating system. Before submitting the device to the U.S. Food and Drug Administration, Damiano plans to start a company to make and distribute it. “There’s a tremendous amount of hope attached to this–for good reason,” says Damiano.
One of the people who have been watching his progress is Fred Cunha, whose daughter Elise, 7, was diagnosed with diabetes when she was 1. Five years later, she became one of the youngest diabetics, at age 6, to enroll in a trial for the bionic pancreas. “In the beginning, I got excited over a lot of solutions for diabetes, but then I became immune,” says Cunha. “For the first time in a long time, I feel like this is something that will actually work.”
But obstacles remain. Not all of the trials are funded yet, and an on-market deadline of 2017 leaves awfully little wiggle room–especially in the world of clinical trials, in which so much can go wrong. Damiano is personally reaching out to the “T1D” community to help him fund the device, and when commercialization plans are under way, the people he wants involved are those with “skin in it,” which is to say, people who either have diabetes or are caring for someone who does. He is convinced that that kind of investment–both financial and personal–will help him meet his goal. But even his most hopeful boosters would settle for less. “Even if it comes out in 2020, I would be ecstatic,” says Cunha.
Affordability is another X factor. Damiano estimates that a bionic pancreas could cost thousands of dollars, not including the additional costs of insulin and glucagon and any maintenance or upgrades to the device. “The bionic pancreas has to be covered [by insurance], or it’s not going to work,” he says flatly.
Proper blood-sugar control is estimated to reduce the risk of eye disease by 63%, kidney disease by 54% and nerve damage by 60% among people with diabetes. Currently, the management of Type 1, including the downstream illnesses it causes, accounts for billions of dollars in health care costs each year.
Even if the bionic pancreas can slash those costs and change patients’ lives, nobody pretends it’s the final answer–or can predict when that answer will be in hand. “I think almost everyone would agree that a cure is a medicine or therapy [a patient] could get on Day One or Two and be done,” says Dr. David Harlan, who is running one of the trials, at the University of Massachusetts Medical School. “We are a long way from that.” Damiano agrees. The bionic pancreas, he concedes, is “a bridge that we can keep extending until there is a cure.”
For now, Damiano is especially driven to complete his work, because only when the device is approved can he offer it to the one person he most wants to help: David. Conflict-of-interest rules prevent him from trying out the device on his child before it’s on the market.
David believes his father will come through. “In a way, I am very happy I was diagnosed at a young age so my dad would be inspired to do this,” he says. “He’s one of the only people who can do it.”
And if all goes well, millions of other people will have good reason to be happy too.
More Must-Reads from TIME
- Donald Trump Is TIME's 2024 Person of the Year
- Why We Chose Trump as Person of the Year
- Is Intermittent Fasting Good or Bad for You?
- The 100 Must-Read Books of 2024
- The 20 Best Christmas TV Episodes
- Column: If Optimism Feels Ridiculous Now, Try Hope
- The Future of Climate Action Is Trade Policy
- Merle Bombardieri Is Helping People Make the Baby Decision
Contact us at letters@time.com