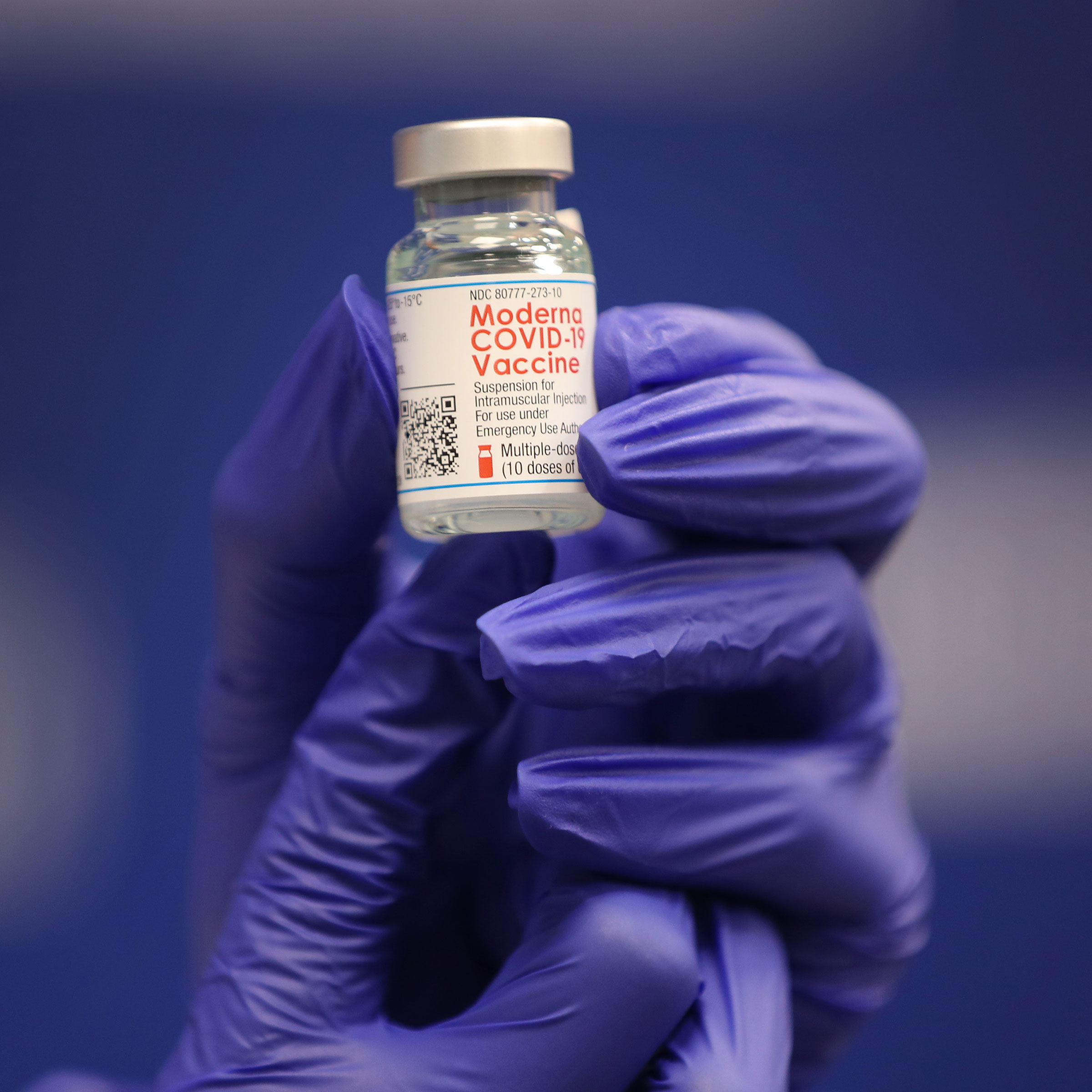
What kind of vaccine is it?
Moderna’s vaccine is based on mRNA technology.
How does it work?
Moderna’s vaccine is the second mRNA vaccine authorized by the U.S. Food and Drug Administration. It relies on the genetic material called mRNA, which codes for proteins. In this vaccine’s case, the mRNA codes for a version of the so-called spike protein that covers the surface of the COVID-19 virus. Once injected in the body, the vaccine causes certain immune cells to produce this viral protein so the body can learn to fight COVID-19 infection in two ways. First, if any cells ever get infected with the virus and carry the spike protein, immune cells can make antibodies that stick to the viral protein like Velcro and neutralize the virus so it can’t make more copies of itself and infect even more cells. Second, killer cells can directly attack and destroy any cells infected with the viral protein.
How effective is it?
According to studies the companies conducted involving more than 30,000 people, the vaccine is 94.5% effective in protecting people from COVID-19 disease. That means that the shot strongly protects people from getting sick with COVID-19, but may not necessarily protect them from getting infected with the virus in the first place. Those studies are still ongoing.
Are there any side effects?
Most of the side effects reported in the clinical trials for the vaccine were mild and relatively rare, including swelling and redness at the injection site, chills and muscle pain. In the Phase 3 study more people reported side effects after the second dose than after the first dose. The U.S. Centers for Disease Control and Prevention are now investigating a couple dozen reports of anaphylactic reactions to both the Pfizer and Moderna shots.
For now, U.K. and U.S. health officials warn that people who are allergic to any of the components of the vaccine should not get the shot.
How is it stored?
The vaccine needs to be stored frozen between -25°C (-13°F) and -15°C (5°F) if it’s not being used immediately. Each vial contains enough doses to vaccinate 10 people.
To use the doses, doctors have to thaw the vials in a refrigerator, usually overnight. Once thawed, the unopened vial needs to be used within 30 days. Once a vial is punctured to get a dose out, it needs to be used within six hours.
Where is it authorized?
The vaccine is not officially approved yet in any country, but authorized in the U.S. and Canada. The company plans to file for full approval when it has gathered more follow up data on people in the ongoing studies.
- Cybersecurity Experts Are Sounding the Alarm on DOGE
- Meet the 2025 Women of the Year
- The Harsh Truth About Disability Inclusion
- Why Do More Young Adults Have Cancer?
- Colman Domingo Leads With Radical Love
- How to Get Better at Doing Things Alone
- Michelle Zauner Stares Down the Darkness