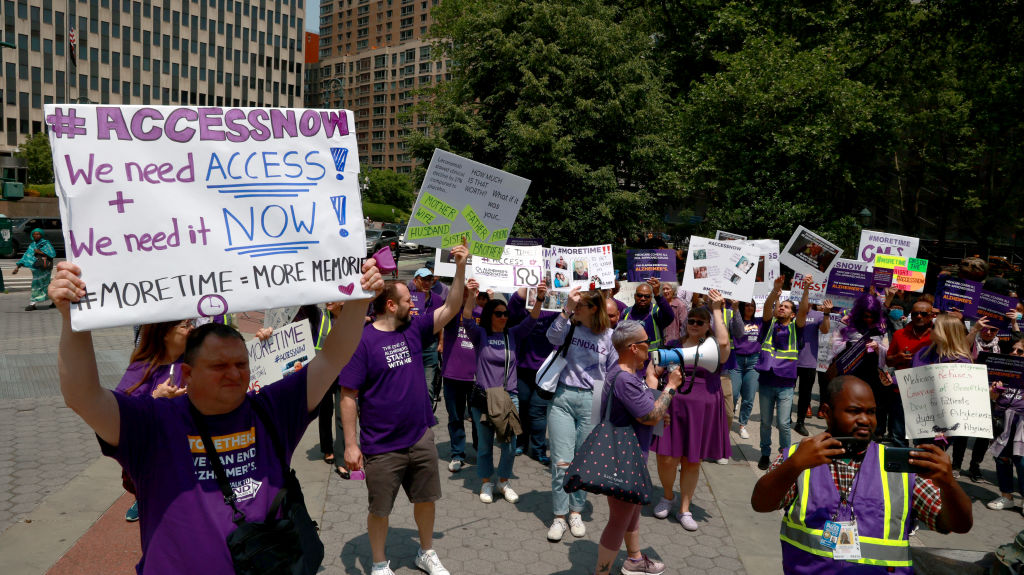It’s a decision that millions of people affected by Alzheimer’s disease and their families have been waiting for—the first fully approved drug that treats the disease, rather than its symptoms. On July 6, the U.S. Food and Drug Administration (FDA) granted full approval for lecanemab, or Leqembi, to treat Alzheimer’s in people in the early, mild stages of the neurodegenerative condition.
Doctors can already prescribe lecanemab, which is made by Eisai Inc. and Biogen, since it has been available under accelerated approval from the FDA since January, but that conditional approval has limited access. The Centers for Medicare and Medicaid Services (CMS), which operates Medicare, decided in response to the accelerated approval that it would not cover the $26,500 yearly cost of lecanemab unless people were part of a clinical trial that continues to gather safety and effectiveness data on the medication. CMS said it will only reimburse for the drug outside of studies if the drug receives full FDA approval, which it now has, and people enroll in a registry that requires doctors to continue to report safety and effectiveness data for their patients using it.
The registry requirement makes the approval a bittersweet victory, say some patient advocates. “It is a hallmark, and something we should be celebrating with champagne dinners,” says Jim Taylor, co-founder of Voices of Alzheimer’s, a patient advocacy group, whose wife Geri was diagnosed with the disease in 2012. “But we can’t until we know whether or not people living with the disease will be able to access it.”
More from TIME
The Alzheimer’s Association has remained adamant that while it supports the need to better understand how lecanemab works in real patients, “traditional approval with the type of data that has been presented by Eisai for Leqembi should never require any type of registry at all,” says Maria Carillo, chief science officer of the Association. “We feel it’s necessary with all treatments to learn more about safety and efficacy. But is it required for coverage? It should not be.”
Within hours of the FDA’s decision, CMS released a link for doctors to enroll in its registry portal, keeping to its previous statements that it would be “easy-to-use” and “wherever possible, drop-down menus will be available.” In a response to questions from TIME about how common such registries are, and whether coverage for other medications have come with similar conditions, a CMS spokesperson cited radiation drugs to treat cancer and devices such as heart valves and stents. In defending the registry, the spokesperson said covering therapies under registry requirements “can expedite earlier beneficiary access to innovative technology while ensuring that systematic patient safeguards, including assurance that the technology is provided to clinically appropriate patients, are in place to reduce the risks inherent to new technologies.”
Based on previous statements, the types of information CMS will require in its registry will be data that doctors are collecting already, including reports of side effects such as brain inflammation and results of cognitive tests to verify the patient’s diagnosis of mild Alzheimer’s disease. CMS has also asked for additional data documenting any meaningful improvement in patients taking the drug, any side effects, and how these benefits and risks change over time with continued use.
More cynical experts say CMS’s decision to require a registry may be driven by the more practical goal of limiting payouts for the first effective Alzheimer’s therapy. While another drug, aducanumab, was approved before lecanemab and also treats the disease, its results were less consistent, and both doctors and patients have been less willing to use it. “Typically when CMS delays things…it’s because of cost concerns,” says Tomas Philipson, emeritus professor of economics at University of Chicago, who previously served in CMS. “The question is, are they staring at total costs or staring just at drug costs?”
In a paper published by the University of Chicago, Philipson has calculated that the health system could save anywhere from $300 billion to $1.8 trillion overall if more people with Alzheimer’s were treated with drugs like lecanemab that can delay progression of their disease. “There is a general misconception, which is common at CMS, that increased drug spending necessarily leads to higher Medicare costs which many times is not true,” says Philipson. “Many times, when you have new drugs, the total health care spending falls because the new drug is offsetting other spending.”
In any case doctors will need to enroll in the registry so their patients can receive Medicare reimbursement for the drug, and in coming months, it will become clear whether the registry becomes an obstacle to access or not. “I do have concerns that if [CMS] makes it too difficult for practitioners not at big academic medical centers—in private practice or those who help underserved communities—then it will further restrict access,” says Dr. Reisa Sperling, director of the center for Alzheimer’s research and treatment at Brigham and Women’s Hospital. For specialists like her, at large medical centers, joining the registry may not be a huge lift. In fact, many groups like hers have already enrolled in the Alzheimer’s Association’s AlzNet, a portal for recording patient experiences with drugs like lecanemab so other physicians can learn from them and better understand how people respond to the medication. It’s not clear whether CMS’s portal will be duplicative of AlzNet or if there are ways to streamline the data collection between the two systems; Carillo says the Alzheimer’s Association plans to discuss this issue with CMS in an upcoming meeting.
Addressing coverage, and therefore access, is critical to better understanding the true impact that disease-modifying drugs like lecanemab can have on Alzheimer’s. “I see this medication as primarily an overdue catalyst for transformational change,” says Dr. Alvaro Pascual-Leone, a professor of neurology at Harvard Medical School and medical director at Hebrew SeniorLife, a non-profit senior health facility in New England. “Dementia care right now is not what it should be. It’s reactive—we wait until people have substantial disability because we don’t do any screening ahead of time,” he says. Having effective medications like lecanemab could spur more doctors to start testing their patients for the first signs of Alzheimer’s dementia, and guide them toward not just drug therapies but lifestyle changes as well, which studies have shown can, in some people, reduce progression of cognitive decline by up to 40%—more than the 27% recorded with lecanemab.
“When we only had symptomatic therapies, there was maybe a certain therapeutic nihilism even on the part of neurologists but also primary care doctors because there wasn’t a great urgency to make a diagnosis,” says Dr. Charles Bernick, a neurologist at the Cleveland Clinic Lou Ruvo Center for Brain Health in Nevada. “But now, with an effective therapy, there is.”
It’s important to set expectations, however, since while lecanemab can slow the continued deterioration of cognitive functions, it cannot improve people’s memory. Essentially, it pushes back the steady decline to buy patients more time during which they can remain independent and able to take care of themselves. Having that time is critical for patients like John Domeck, a retired attorney in Aurora, Ohio, who was diagnosed with Alzheimer’s in 2019. His doctor was honest about his outlook, telling him and his wife Ann that he would have about eight years before the more severe symptoms of the condition started to appear, including problems with his speech, physical clumsiness, more serious memory loss and finally not being able to swallow. For nearly a year they focused on lifestyle activities that can slow Alzheimer’s—reading, exercising, doing puzzles, and trying to stay socially engaged, despite the COVID-19 lockdown. “We sat in our driveway and talked to our neighbors,” says Ann. In 2020, Domeck joined the trial for lecanemab, and for 18 months he and Ann did not know if was getting the drug or placebo twice a month. After the study ended, he began receiving the drug for certain in July 2022, in the open-label portion of the trial, which will continue for another two years or so. “I noticed a difference,” says Ann of her husband’s short-term memory improvements since last year. At a beach gathering with relatives two weeks ago, she says John remembered the drinks that were served during their past family Christmas celebration. “That struck me,” she says. “Short-term things he would never remember before, he does now.” Slowing the disease has allowed them to continue traveling, and for John, keep playing golf.

John is now testing a more convenient way to deliver lecanemab, with autoinjections that Ann gives him in the abdomen once a week at home instead of the twice-monthly IV infusions at the Cleveland Clinic, where he was part of the study. Eisai says the company is planning to report results of the self-injections by the end of the year.
John never hesitated about volunteering, and says “to participate in the trial was something we both looked forward to.” With lecanemab’s approval, he and Ann are beginning to appreciate what that decision could mean for Alzheimer’s patients. “I don’t think we understood what would come out of it,” says Ann. “But the more we were in it, the more we got the impact. This is amazing.”
More Must-Reads from TIME
- Cybersecurity Experts Are Sounding the Alarm on DOGE
- Meet the 2025 Women of the Year
- The Harsh Truth About Disability Inclusion
- Why Do More Young Adults Have Cancer?
- Colman Domingo Leads With Radical Love
- How to Get Better at Doing Things Alone
- Michelle Zauner Stares Down the Darkness
Contact us at letters@time.com