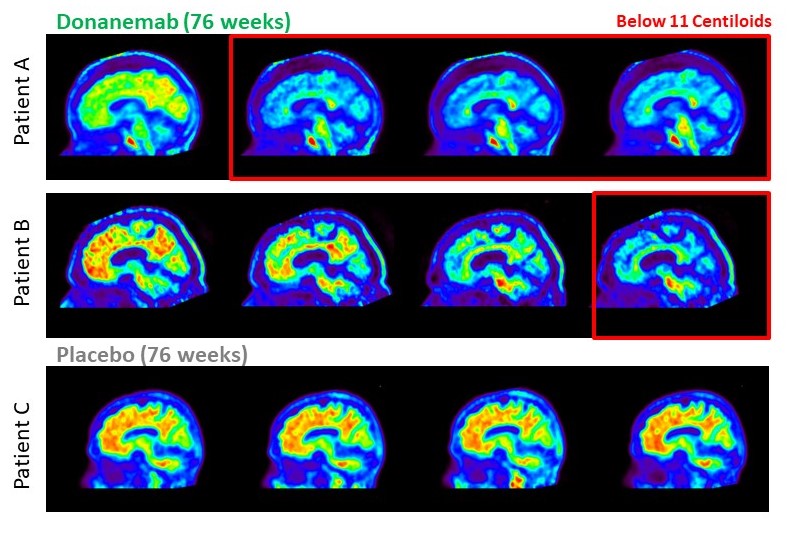
In a May 3 press release, Eli Lilly announced encouraging results from its latest study of its Alzheimer’s drug candidate, donanemab.
In the Phase 3 study, which included more than 1,000 people with early signs of Alzheimer’s disease, people receiving donanemab experienced 35% slower cognitive decline, as measured on cognitive tests, than people receiving placebo. In addition, those getting the drug showed 40% less decline in the ability to perform daily activities such as driving, managing their finances, and holding conversations. That’s an important metric for patients and their caregivers, as it can help more people live as independently as possible for as long as possible as the disease progresses.
The results are the most encouraging yet for any drug that targets amyloid, the protein that builds up in the brains of Alzheimer’s patients and leads to a gradual culling of nerves involved in memory and other cognitive skills. “This further underscores the inflection point we are at for the Alzheimer’s field,” said Maria Carrillo, chief science officer for the Alzheimer’s Association, in a statement. “The progress we’ve seen in this class of treatments, as well as the diversification of potential new therapies over the past few years, provides hope to those impacted by this devastating disease.”
More from TIME
Read More: A New Alzheimer’s Drug Gave Patients Hope. Millions Can’t Get It
The only drugs currently approved to treat Alzheimer’s—aducanumab and lecanemab—slow cognitive decline to a lesser extent, and early promising studies on donanemab had Alzheimer’s experts hopeful that Eli Lilly’s drug would benefit patients to a greater extent. The company plans to request approval from the U.S. Food and Drug Administration (FDA) for donanemab for early Alzheimer’s patients this quarter, and will file additional applications for broader global approval.
But even if does gain FDA approval, access to the medication could be an issue. Aducanumab, the first drug approved to treat Alzheimer’s, isn’t widely available, since the Centers for Medicare and Medicaid Services (CMS) decided it would only reimburse patients who use the drug if they are enrolled in a clinical study to continue researching the effectiveness of the medication. Coverage for lecanemab, the second recently approved drug, is also restricted by CMS, since it also works by targeting amyloid.(The Veterans Affairs Administration, however, does cover lecanemab for its beneficiaries.)
CMS says it restricts coverage of anti-amyloid medications because these medications require further study before Medicare and Medicaid can reimburse all users. Under that policy, coverage of donanemab, despite its strong positive effect, could also be limited. “The Centers for Medicare & Medicaid Services (CMS) policy to block Medicare access to Food and Drug Administration (FDA)-approved Alzheimer’s treatments is in stark contrast to scientific evidence, is unprecedented and must be reversed immediately,” said Joanne Pike, president and CEO of the Alzheimer’s Association, in a statement.
More Must-Reads from TIME
- Cybersecurity Experts Are Sounding the Alarm on DOGE
- Meet the 2025 Women of the Year
- The Harsh Truth About Disability Inclusion
- Why Do More Young Adults Have Cancer?
- Colman Domingo Leads With Radical Love
- How to Get Better at Doing Things Alone
- Michelle Zauner Stares Down the Darkness
Contact us at letters@time.com