The U.S. Food and Drug Administration (FDA) just issued its first emergency use authorization for an at-home test that can tell users if they have either the flu, COVID-19, or both.
The decision on Feb. 24 comes as more people become comfortable with the idea of testing themselves at home to get quick diagnoses for COVID-19. Public health officials have also increasingly warmed up to at-home testing during the COVID-19 pandemic.
But with so many overlapping symptoms between COVID-19 and the flu, doctors and the public have used a COVID-19 rapid test to rule out a SARS-COV-2 infection. If it’s negative, they might assume symptoms are from influenza.
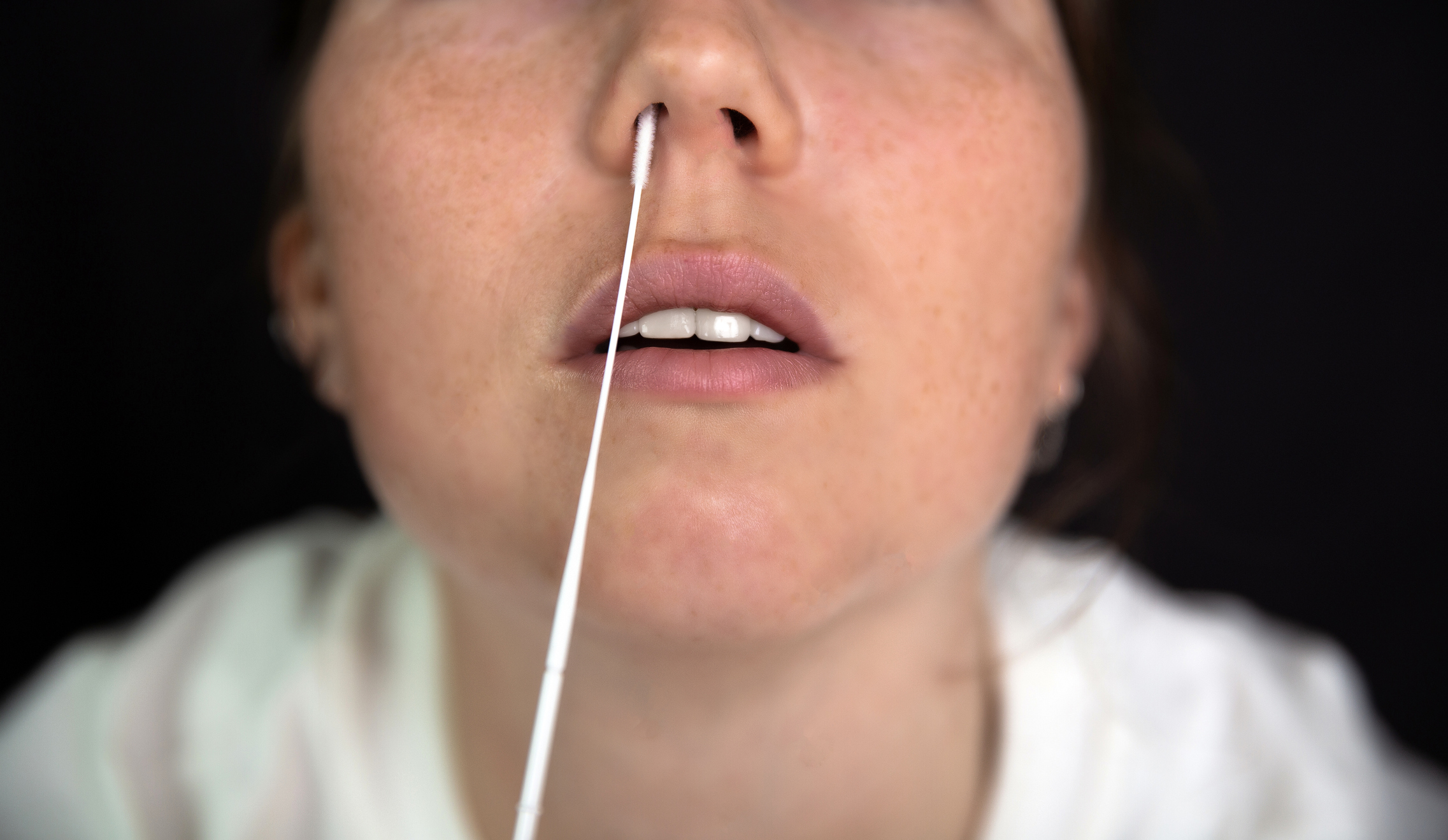
Now you can understand what is causing your symptoms with more certainty. The FDA’s emergency use authorization for the test, made by Lucira, works in the same way as the at-home rapid antigen tests for COVID-19. People take the provided swab in the kit to swab their nose, then place the tip in a solution that provides results in about 30 minutes. The test is designed to pick up two types of influenza viruses, A and B, and SARS-CoV-2, the virus that causes COVID-19.
The tests are expected to be rolled out soon, and are available without a prescription at the same locations where at-home COVID-19 tests are distributed.
According to the data the FDA reviewed, the test correctly detected 90.1% of positive influenza A samples, 99.9% of negative influenza B, and 88.3% of positive COVID-19 samples. There are currently not enough cases of influenza B for the study, so the FDA is requiring Lucira to continue documenting the test’s ability to detect that type of flu once the test is available to the public.
Knowing which virus you have can speed treatment—influenza and COVID-19 are caused by different kinds of viruses and therefore treated with different antiviral therapies.
The FDA urges people to share their results with their health care provider, whether positive or negative, in order to receive the most appropriate treatment. If both tests are negative, then doctors can test for and treat other infectious diseases.
The double test is an indication that health officials are reassured by the uptake and use of the COVID-19 self tests, which were the first for an infectious disease, and are willing to consider broadening the array of tests that people can administer on their own.
Other home tests that combine flu, COVID-19 and even RSV—another infectious disease that primarily affects young children and the elderly—are also on the horizon.
More Must-Reads from TIME
- Donald Trump Is TIME's 2024 Person of the Year
- Why We Chose Trump as Person of the Year
- Is Intermittent Fasting Good or Bad for You?
- The 100 Must-Read Books of 2024
- The 20 Best Christmas TV Episodes
- Column: If Optimism Feels Ridiculous Now, Try Hope
- The Future of Climate Action Is Trade Policy
- Merle Bombardieri Is Helping People Make the Baby Decision
Contact us at letters@time.com