In a ground-breaking experiment, researchers have successfully created the first human-monkey chimera. The work, published in the journal Cell, describes the the first embryo containing both human and monkey cells that was cultured for 20 days. Led by Juan Carlos Izpisua Belmonte, the study represents the culmination of decades of work in understanding early embryo development in non-human species, which Belmonte hopes will now apply to humans. But it is bound to raise serious ethical questions about the implications of combining human cells with those from a different species (even if it is a closely related one), and the report was accompanied by commentary from ethicists on how the work should be interpreted and what the careful next steps should be in pursuing this line of study.
Belmonte, a professor in the gene expression laboratory at the Salk Institute for Biological Studies and well-respected for his work in embryo development, is very clear about why he pursued the experiment, and where he hopes it will lead. Creating cross-species mutants to capitalize on specific physical features or characteristics, X-Men style, was most definitely not the goal. In interviews with TIME on the experiment as it progressed since 2019, he carefully laid out the biological mysteries he hoped to solve, and the gaps in knowledge about early development he wanted to fill.
The earliest steps that human embryos take, he says, are the “biggest mystery of human development. While we know a lot about development after we are born, and even a little about what happens during pregnancy, we really don’t know anything about human development in the first two or three weeks after fertilization. All we know about early embryogenesis comes from different lab models—from rodents, mice and worms—but we really don’t know anything about us.”
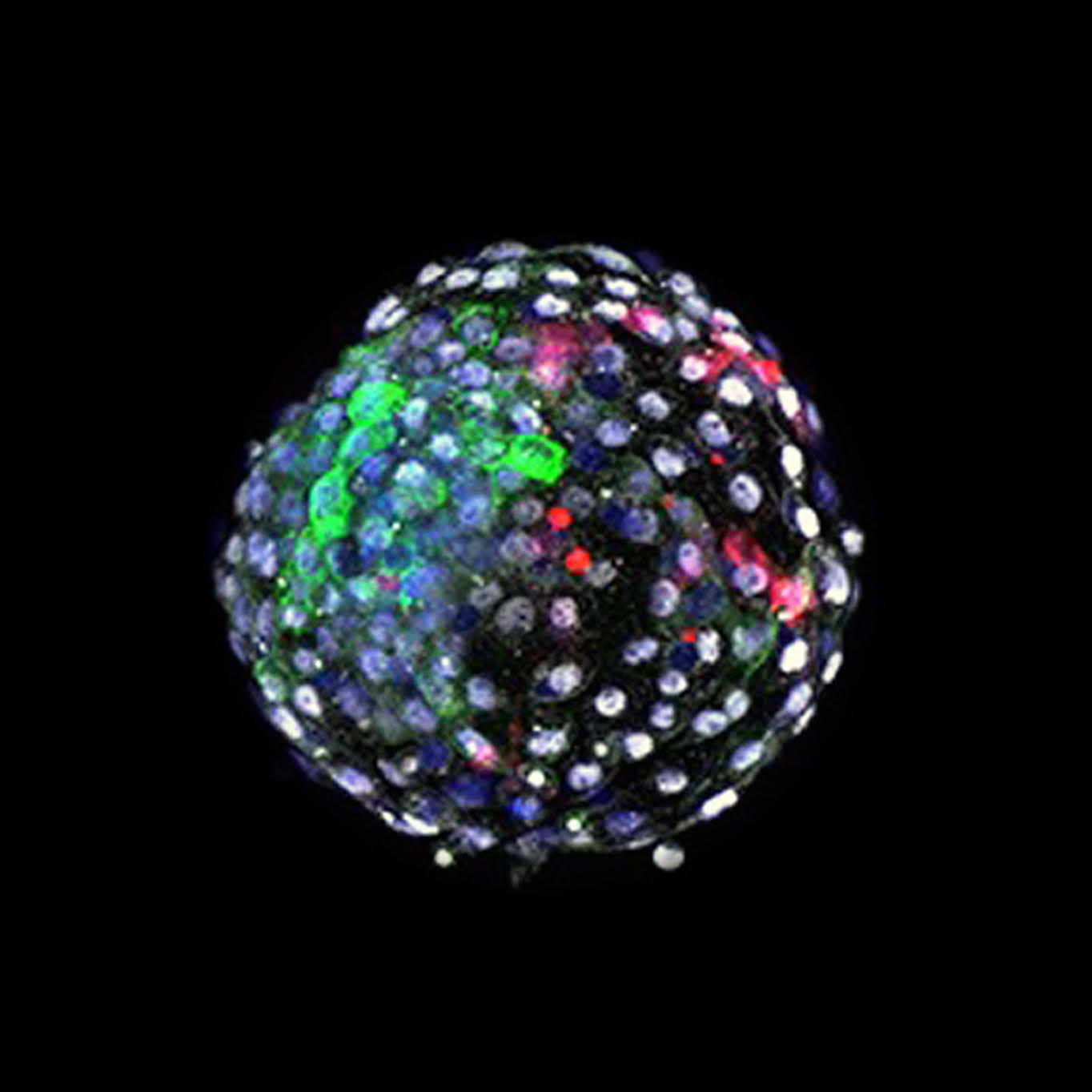
In the Cell paper, Belmonte describes creating a human-monkey chimera that is viable for 20 days, enough time to study these critical early stages of development. Specifically, he and his team were looking for the different signals that the nascent cells send out to spark development from a single fertilized cell into the millions of cells and multiple tissues and organs that comprise a human. The evolutionary closeness between primates and humans makes this possible—Belmonte previously tried similar experiments with human and pig embryos, and failed.
He is fully aware of the delicate moral and ethical questions his work stirs up: “We are not going to use monkeys to create human organs inside monkeys,” he says, referencing one of the potential outcomes of such research that many feel crosses ethical lines.
Instead, Belmonte plans to learn the “language” of early human embryo development using this human-monkey model, and then use that to better understand disease—and potentially generate human tissues for organ transplantation in a less ethically charged species such as the pig, which is more distant from humans evolutionarily and more accessible in terms of conducting experiments.
Learning from the animal world
You’re likely to be struck by an odd juxtaposition when you set foot on the campus of the Salk Institute for Biological Studies in La Jolla, Calif., a seaside neighborhood in San Diego. On the outside, there doesn’t seem to be much happening in the two massive six-story concrete edifices that stretch from the entrance gate until they reach the cliffside, where they seem to drop off, infinity-pool fashion, into the Pacific Ocean beyond. The bleached concrete and travertine courtyard of the research institute is pristine, evoking calm and simplicity, in accordance with the directive of its founder, Jonas Salk, famous for developing the polio vaccine. Built in 1960, the facility is now as well known for its placid, soaring architecture as for the boundary-pushing science that happens in its labs.
Belmonte’s lab occupies a corner of the more than 411,000-square-foot Institute. Like his surroundings, the Spanish-born scientist is reserved and unruffled, and speaks softly—never hinting at the fanfare-deserving scientific and ethical boundaries he pushes every day with his work. Belmonte has been fascinated with the first sparks of life—what scientists call “embryogenesis,” or how embryos develop—his entire career. As a newly minted PhD in 1987, Belmonte published one of his first scientific papers, reporting the results of a study in which he took embryonic cells from a mouse limb and grafted them onto the beginnings of a wing of a chicken embryo. To his surprise, the mouse limb cells developed well in their new environment, suggesting that embryonic cells from different species could, at the least, talk to each other—opening the door to growing tissues or organs of one species in another one.
More than 30 years later, Belmonte still repeats the mantra his mentor, renowned British developmental biologist Lewis Wolpert, liked to tell his students: Forget birth, marriage, or death. The most important moment in your life is an event that occurs when you are still an embryo.
That moment is called gastrulation, and for humans, it occurs about two weeks after egg and sperm meet in fertilization. It’s when the embryo, which began as a single egg—then divided to become two cells, then four cells, then eight and so on—splits into the three major cell layers that eventually give rise to the more than 200 cell types that make up the human. That’s the lightning-rod moment—when brain cells start to distinguish themselves from skin cells and lung cells diverge from hair cells, and so on.
However important this moment, it’s currently challenging to study. Guidelines from U.S. and international scientific organizations advise researchers not to keep human embryos dividing in the lab beyond about 14 days. That’s to forestall ethical concerns about potentially growing a human embryo that will start to develop cells, tissues and organs. Because human gastrulation occurs during the third week, the critical steps leading to this milestone have, to date, remained a black box.
Intent on figuring out a way to shed more light on this crucial window of time, Belmonte first spent years trying to understand how salamanders and amphibians regenerate not just limbs but other more complex organs and structures like spines and brains—which, like gastrulation, is a unique form of development. He narrowed down the key genes responsible for instructing tabula rasa cells to become other types of cells, but the animals were still many steps removed from humans.
In the early 1990s, when researchers first isolated human embryonic stem cells, Belmonte saw an opportunity to finally break through the secrets of early human development. Ethical issues about working with these early cells, however, stymied the field until 2007, when a Japanese scientist, Shinya Yamanaka, figured out a way to chemically reprogram fully developed human cells to revert back to an embryonic-like state, so they can start developing all over again.
Yamanaka earned the Nobel Prize in 2012 for his groundbreaking work, but his process, while innovative, wasn’t very reliable and still too nascent to answer the questions Belmonte wanted to address. So he thought: Why not mimic the entire human development process by piggybacking off another animal species? Thinking back on his early chimera experiments, Belmonte successfully merged mouse cells in rat embryos and vice versa, and was eager to see if a similar model could be created with human cells, so he could study those out-of-reach first critical stages of human development.
Unfortunately, when he tried to grow human cells in pig and sheep embryos, he hit a wall. A small percentage of the human cells did find a way to survive, but, by and large, the pig and sheep embryos rejected the human cells—not surprising since the evolutionary gap between pigs and sheep and humans is much greater than that between mice and rats. Belmonte knew he needed a better model.
Unlocking the secrets of early human development
The problem was that non-human primates, the animals closest to humans on the evolutionary tree, proved to be almost as tricky as humans when it came to culturing and nurturing embryos at their earliest stages.
First were the funding issues. The National Institutes of Health, the largest source of basic biomedical research funding in the U.S., does not support human chimera studies. So Belmonte turned to his native Spain, where such work is allowed, as well as private foundations and new resources such as the state-funded California Institute for Regenerative Medicine.
Next were the biological hurdles. Until 2019, no non-human primate embryos had been cultured for the nearly three weeks it took for them to reach the gastrulation stage that Belmonte was keen to better understand. That year, however, Belmonte collaborated with colleagues led by Weizhi Ji at the Primate Biomedical Research in Kunming, China to successfully culture monkey embryos to 20 days, just when the critical phases of gastrulation start to take shape.
The study, published in the journal Science barely caused a ripple in the larger world, which soon became subsumed by the COVID-19 pandemic. But the advance was critical for Belmonte’s chimera aspirations. With a monkey embryo viable to 20 days, he could start introducing human cells into that embryo and explore the first flurry of genetic, molecular and chemical changes that dictated early development.
The results of this work—mostly done just before Belmonte’s labs at Salk were locked down due to the pandemic from March to June—were published in Cell on April 15. What the years-in-the-making study revealed elicits impassioned excitement from even this soft-spoken scientist. “I was surprised, although I had hoped this would work much better than the human-pig studies,” he says, a broad grin spreading across his face. “It was like seeing evolution happening in front of your eyes.”
What Belmonte saw was the remarkable mingling of monkey cells with human cells he had introduced into a monkey embryo as it developed. The human cells came from a stem cell line in China, using Yamanaka’s method of reprogramming an adult cell back to its embryonic state. Twenty-five of these reprogrammed human cells were introduced into each of 132 monkey embryos. With each day, fewer of the embryos remained viable, and by day 19, only three remained. Belmonte admits that this first pass wasn’t a resounding success, though he is hesitant to definitively calculate the efficiency of the process, given that this study entailed just a single injection of human cells at a single time point; previous experiments with mouse and rat cells involved a range of numbers of cells introduced at a range of time points during embryonic development. “We only did one number of cells at one specific time,” he says. “Any conclusion in terms of efficiency would not be valid at all. But the fact that these cells communicate and develop more or less normally clearly says the efficiency has to be high. We will next do more experiments, and vary the number of cells and time periods to answer the efficiency question more precisely.”
Nevertheless, this first foray into human-primate chimeras does prove that cells from multiple species can talk to each other—which means Belmonte’s vision of having a model, ex vivo, or outside of the body, for studying the very first stages of human development, could become reality.
And, once he identifies the signals and processes that human cells use to differentiate into different tissues and organs, he can recreate that environment in pig embryos, and ultimately regenerate human tissues such as skin grafts for burn patients and heart, lung or liver tissue to replace damaged and diseased cells.
Science, not science-fiction
Belmonte is already collaborating with scientists and pig farmers in his native Spain to set up the next studies. He is well aware that his work is controversial and will strike some as breaking the laws of nature. In preparing for his human-monkey chimera work, he consulted three independent ethicists and obtained approval from the institutional review boards that oversee any studies involving people or human tissues, from not just the Salk Institute but his collaborators’ institute in China as well.
“That’s unheard of for biomedical science,” says Insoo Hyun, a bioethicist at Case Western Reserve University and director of research ethics at Harvard Medical School. “They went above and beyond what people typically do for ethical oversight, and that attests to the fact that everybody understands that the use of non-human primate embryos is ethically sensitive because they are [evolutionarily] so close to man.”
Hank Greely, director of the Center for Law and the Biosciences at Stanford University, who wrote an editorial accompanying the study, says that he has “no real problem” with the experiment itself, because the embryo will not be implanted for further gestation. But he recognizes that such experiments can alarm people, and be misunderstood if not explained properly. One concern people might have, notes Greely, is about consent for such studies; it’s important he says, that cell donors are fully informed that their cells might be used for chimeric research. Most informed consent forms cover the typical studies for which such cells might be used, but may not accommodate more cutting-edge applications such as this one, since they couldn’t have been envisioned when the cell lines were donated and made.
“Whoever’s cells led to the cell line that was used, did that person know it would be used for this kind of chimeric research, and should that be part of the consent process?” says Greely. “That’s one of the hottest issues right now in research ethics—whether certain kinds of research are so controversial, that they either require or should involve some endeavor to get specific consent to them—and [that issue] is unresolved.”
The human cells used in Belmonte’s research come from a stem-cell line created and approved by the ethics committee at Peking University, and were part of previous studies Belmonte and his colleagues in China did that led to this work. While both review boards of those universities approved the consent and the study, it’s not clear if the consent included specific mention of the chimera research.
Even apart from that issue, he says, people may not have the same ethical comfort as he does with the scientific merits of the study. “Where the public gets most concerned is when something comes out of the blue that has frightening implications that they had no preparation for,” says Belmonte. “Frankly I think science could do more prep work such as having more public discussion before doing predictably controversial work.”
Hyun, who chairs the International Society for Stem Cell Research committee that is currently writing guidelines for human-chimera studies agrees, says there are no existing ways to have such a dialogue. “Without a forum for the public to get more information about studies like these, and to express their concerns, I think we are heading toward a dangerous place of lack of public support and distrust in science,” he says.
There are no easy solutions, Hyun says, but the town-hall model seen in, for example, U.S. presidential election campaigns, could be a model. In this case, Hyun says, he’d want to see such a town hall hosted by trusted organizations such as the National Institutes of Health or the National Academy of Sciences. It could, he argues, be a starting point for critical conversations where scientists and the public can exchange ideas and concerns and potentially find common ground. “Scientists tend to have a different world view of human beings and their relation to nature than other people who don’t share a scientific orientation,” he says. “Scientists tend not to see a sharp distinction between human and non-human species, since we share genes in common; they tend to see species more as a continuum. Whereas the public see human beings and non-human beings, and they don’t mix. That’s why public dialogue would be helpful, so scientists can appreciate other points of view and the public can appreciate how scientists understand the world.”
Belmonte is eager to participate in such discussions. He was, he says, unfairly criticized when premature news of his work was leaked to the Spanish publication El Pais by one of his collaborators at Murcia Catholic University in Spain, and taken out of context by some who did not understand the purpose of the work. “We need to not do every experiment that we can do, but move forward in ways that are legally and ethically allowed,” he says.
All that said, Belmonte is eager to find out where his latest advance can go next. He believes his work isn’t limited to an academic understanding of early human development, or even to the already sci-fi-level possibility of regenerating human tissue for organ transplants—it can, he hopes, finally expose how certain diseases, even those associated with aging, such as cancer and Alzheimer’s, get started. By placing specific types of cells into a human-primate chimera, scientists can start to understand and use the process of elimination to isolate the precise cells, signals and pathways that can go awry in disease. “So it’s not just the earliest stages of life but also the later stages of our life that could be studied with these chimeric platforms,” he says. That is, as long as our cultural and societal norms can catch up to the fast pace of scientific progress.
More Must-Reads from TIME
- Cybersecurity Experts Are Sounding the Alarm on DOGE
- Meet the 2025 Women of the Year
- The Harsh Truth About Disability Inclusion
- Why Do More Young Adults Have Cancer?
- Colman Domingo Leads With Radical Love
- How to Get Better at Doing Things Alone
- Michelle Zauner Stares Down the Darkness
Contact us at letters@time.com