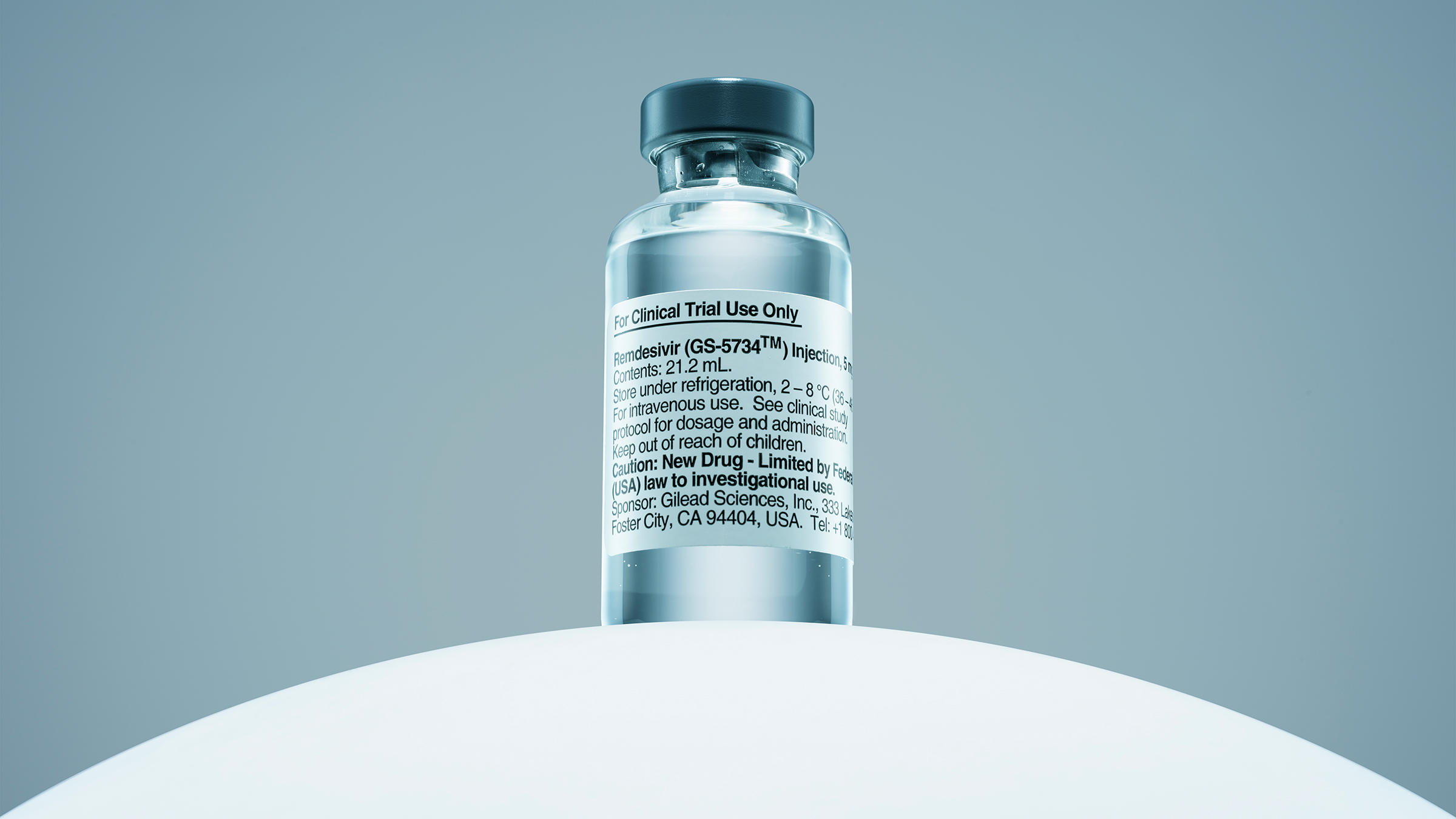
On June 22, Gilead, the California-based pharmaceutical company that makes remdesivir, said it will start testing an inhaled form of its experimental COVID-19 therapy. Currently, patients can only receive the drug by IV infusion provided under the supervision of medical experts.
In a letter published to the company’s website, chairman and CEO David O’Day said Gilead will begin screening healthy volunteers this week to participate in a phase 1 trial of a nasally administered version of remdesivir. Phase 1 trials are designed to determine the safety of an experimental treatment, before moving on to studies of its effectiveness.
The IV-administered form of remdesivir is still being tested, along with other drugs, among the sickest patients who are hospitalized for COVID-19. Early data from those studies suggest that remdesivir can help patients recover more quickly compared to patients not receiving the infusions. Because the drug is not yet approved; patients can only receive it either by participating in a trial or, if they are severely ill, through an emergency use authorization from the U.S. Food and Drug Administration (which allows doctors to prescribe the drug if no other therapies have worked).
The company also announced in its June 22 letter that it will investigate using remdesivir in COVID-19 patients with less severe symptoms, in the hopes that introducing the drug earlier in the disease may help some people avoid the worst symptoms of respiratory distress and failure.
More Must-Reads From TIME
- The 100 Most Influential People of 2024
- Coco Gauff Is Playing for Herself Now
- Scenes From Pro-Palestinian Encampments Across U.S. Universities
- 6 Compliments That Land Every Time
- If You're Dating Right Now , You're Brave: Column
- The AI That Could Heal a Divided Internet
- Fallout Is a Brilliant Model for the Future of Video Game Adaptations
- Want Weekly Recs on What to Watch, Read, and More? Sign Up for Worth Your Time
Contact us at letters@time.com