From her laboratory in the far western reaches of Montana, Elizabeth Fischer is trying to help people see what they’re up against in COVID-19.
Over the past three decades, Fischer, 58, and her team at the Rocky Mountain Laboratories, part of the National Institutes of Health’s (NIH) National Institute of Allergy and Infectious Diseases, have captured and created some of the more dramatic images of the world’s most dangerous pathogens.
“I like to get images out there to try to convey that this is an entity, to try to demystify it, so this is something more tangible for people,” says Fischer, one of the country’s leading electron microscopists.
Now, as her renderings of the coronavirus flash across screens worldwide, she says: “You often hear people call it the invisible enemy. It’s trying to put that face out there.” Working in one of the nation’s 13 “Biosafety Level 4” labs—those equipped to safely handle the most dangerous pathogens—Fischer and her team visualize the world’s deadliest plagues from Ebola to HIV, salmonella to SARS-CoV-2, the coronavirus that causes COVID-19.
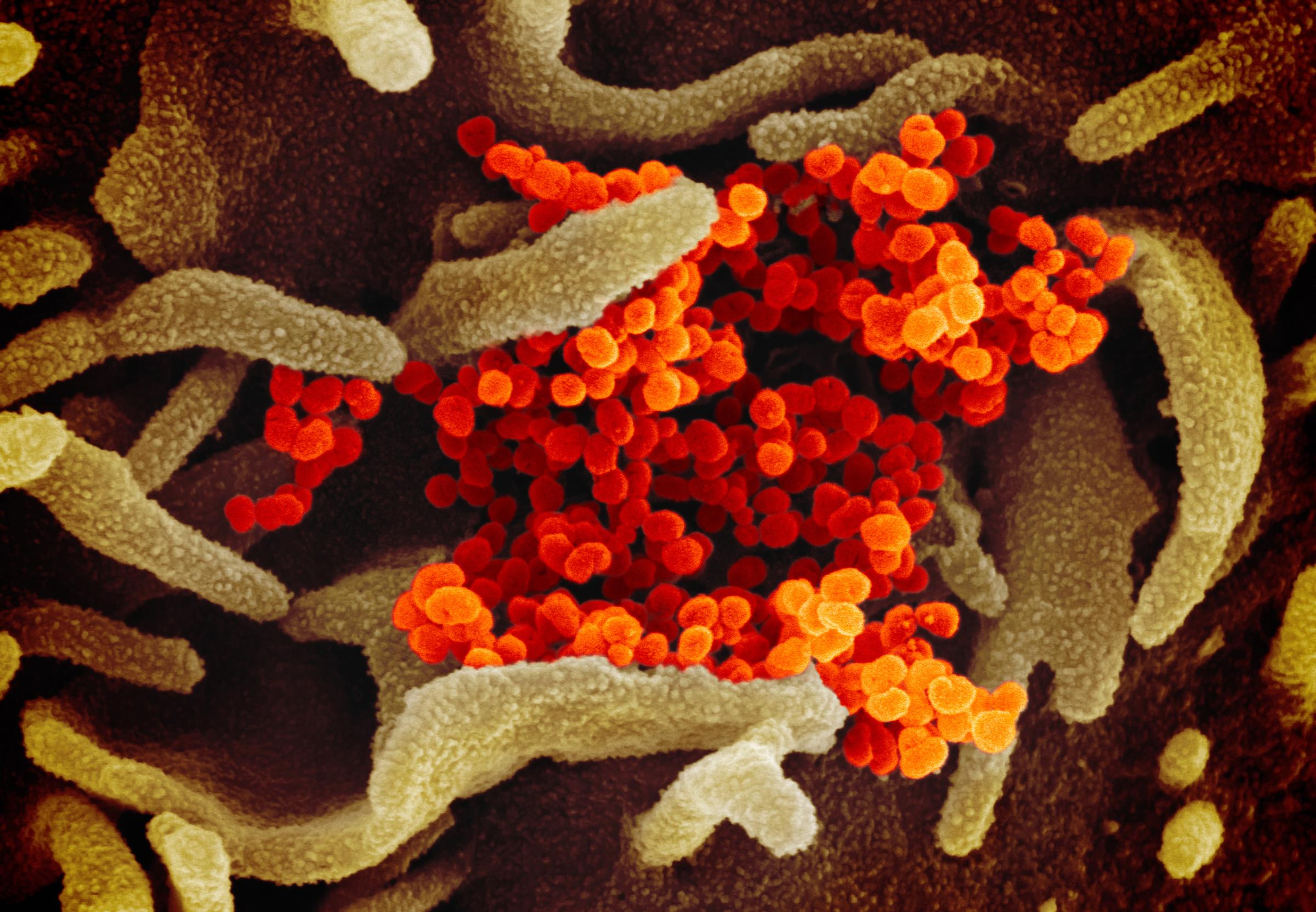
The breathtaking images allow people to see a virus as elaborate biological structures with weaknesses that can be exploited, yielding clues for researchers about how to develop treatments and vaccines.
Originally from Evergreen, Col., Fischer completed a degree in biology at the University of Colorado-Boulder and contemplated going to medical school, before deciding instead to join the Peace Corps. She taught math and science for two years in Liberia, and then took time to travel through East Africa and Asia, including a trek into the Himalayas. Returning to Colorado, she immersed herself in the outdoor world she loved. She worked as a rafting guide on the Arkansas River for several summers, and as a children’s ski instructor at the Monarch Mountain ski resort during the winters.
She later enrolled in graduate school to study education, thinking she might teach biology. But when she took courses in electron microscopy, she was hooked.
It appealed to her sense of exotic adventure. “You’re looking at a world that most people don’t get to see,” she says. She switched gears and completed a master’s degree in biology.
Upon graduation, she sent her resume to a national microscopy job placement office and soon received a call from Rocky Mountain Laboratories. In 1994, she moved with her family to Hamilton, a city of fewer than 5,000 people about 50 miles south of Missoula, then worked her way up to become chief of the lab’s microscopy unit.
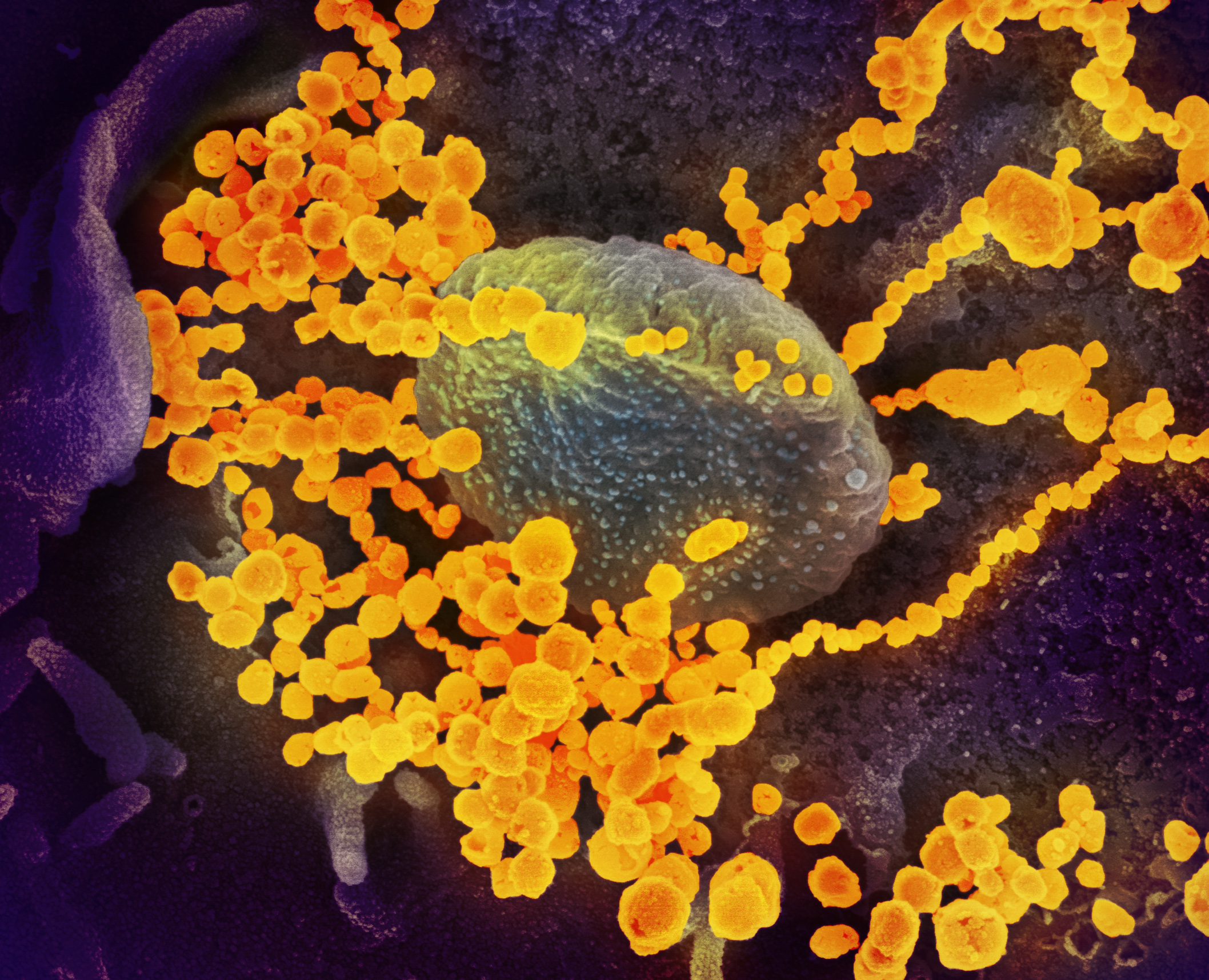
Some of the more stunning images of the coronavirus—about 10,000 times smaller than the width of a human hair—have come from Fischer’s microscope. One is Fischer’s photograph of viral particles being released from a dying cell infected with the virus.
As NIH director Dr. Francis Collins recently highlighted in his blog, the photo shows the orange-brown folds and protrusion on the surface of a primate’s kidney cell infected with SARS-CoV-2. The dozens of small, blue spheres emerging from the surface are the virus particles themselves. (The images produced by the electron microscopes are black-and-white, so Fischer hands them over to visual artists who colorize the image to help identify different parts of the cell and to distinguish the virus from its host.)
“This image gives us a window into how devastatingly effective SARS-CoV-2 appears to be at co-opting a host’s cellular machinery,” Collins wrote. “Just one infected cell is capable of releasing thousands of new virus particles that can, in turn, be transmitted to others.”
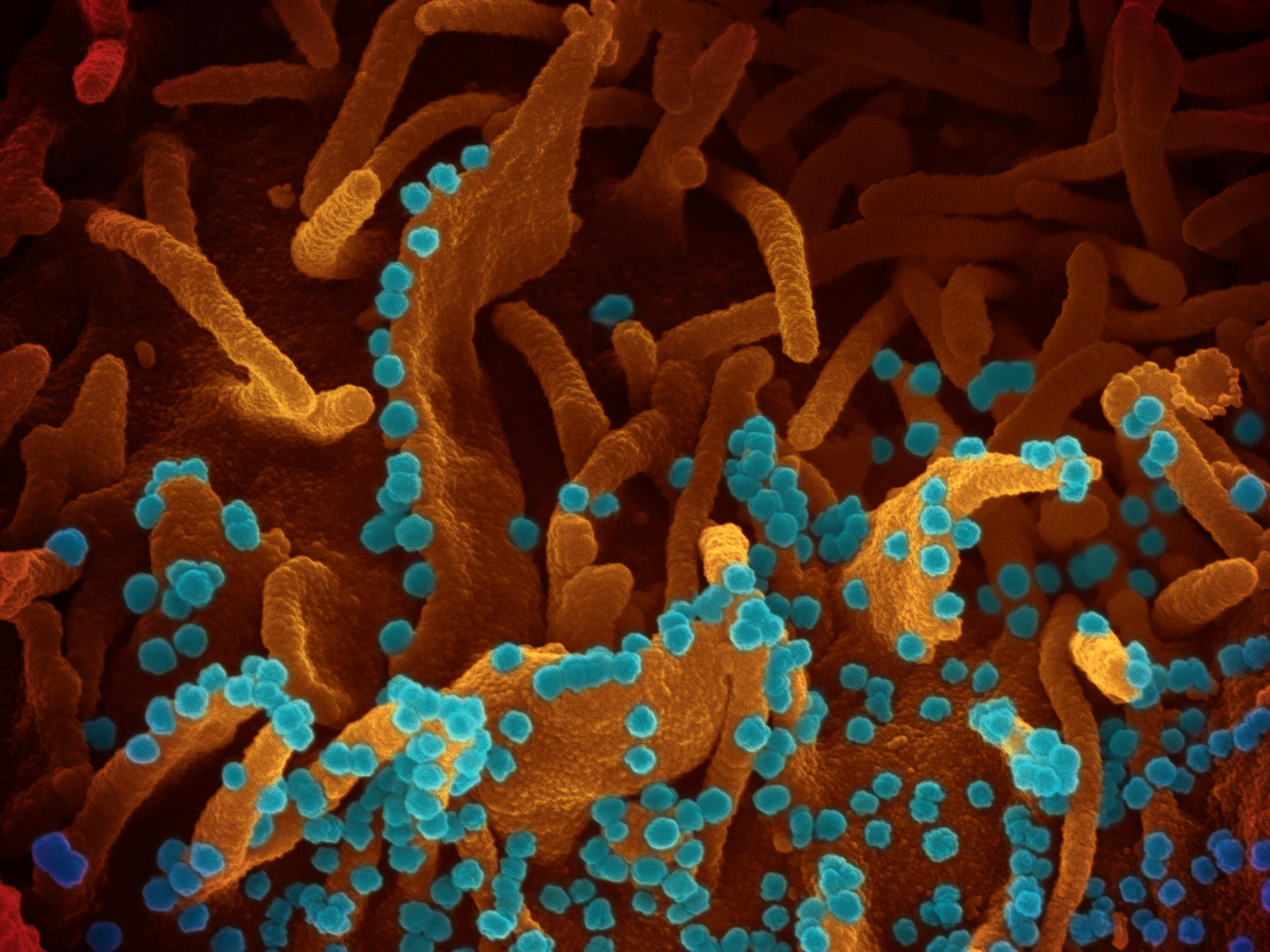
Scientists like Fischer have used electron microscopes to uncover the unseen world of viruses and bacteria dating to the 1930s. In the past two decades, however, new technologies have unleashed a resolution revolution, allowing researchers to see down to the near-atomic level. Microscopists have come up with better ways to prepare samples for viewing and have written sophisticated software programs to sharpen images.
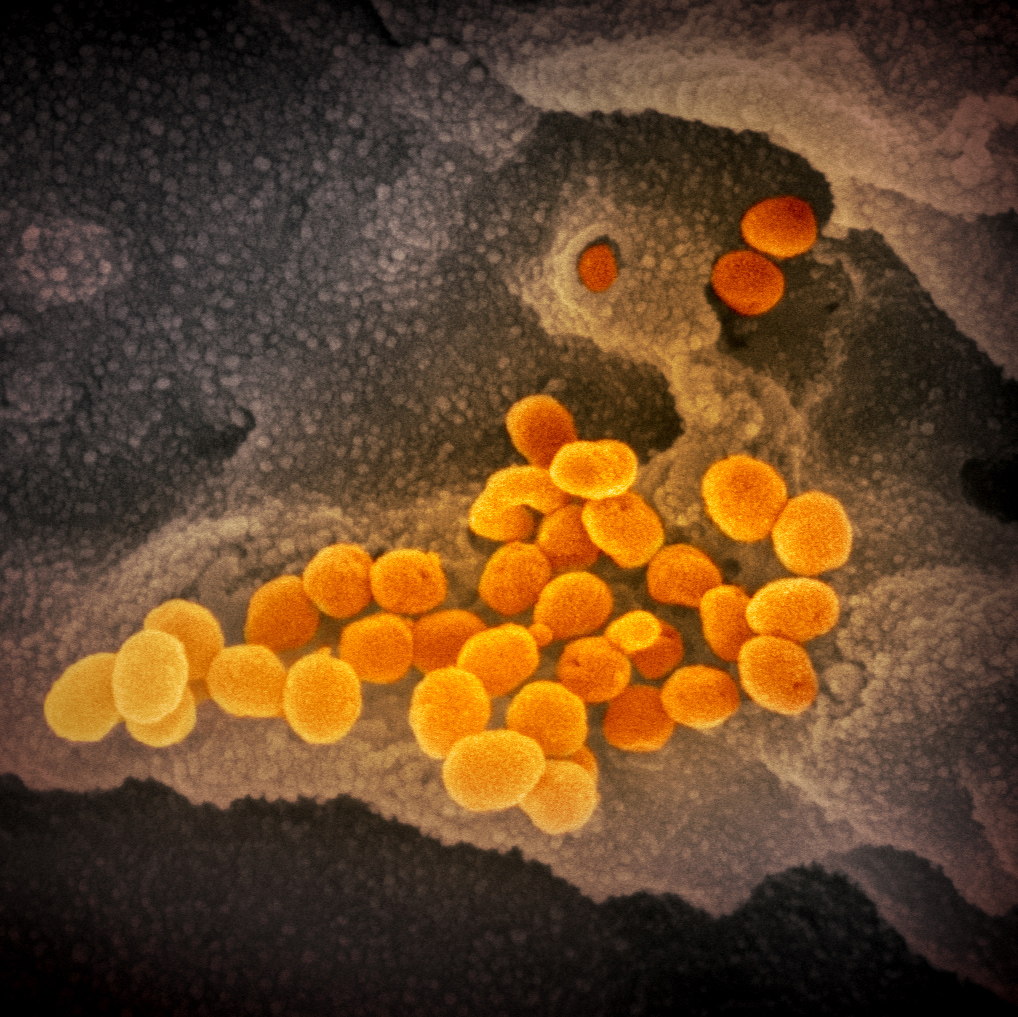
Through her lab, Fischer receives samples from all over the world, and was sent viral material from SARS-CoV-2 in early February from one of the first U.S. patients to be infected. Often, her samples come from vials that have been stored in a freezer for decades, or from cultures routinely grown in a lab. “It’s very sobering when you know it came from a human patient.”
For example, in 2014, a sister lab in Mali sent over an Ebola sample from a 2-year-old girl who had lived in Guinea when her mother died of the disease. Her grandmother traveled from Mali to attend the funeral, which involved touching and bathing the body, and to take the girl home with her. Both got infected and brought the virus back with them as they returned to Mali by public transportation. They both died.
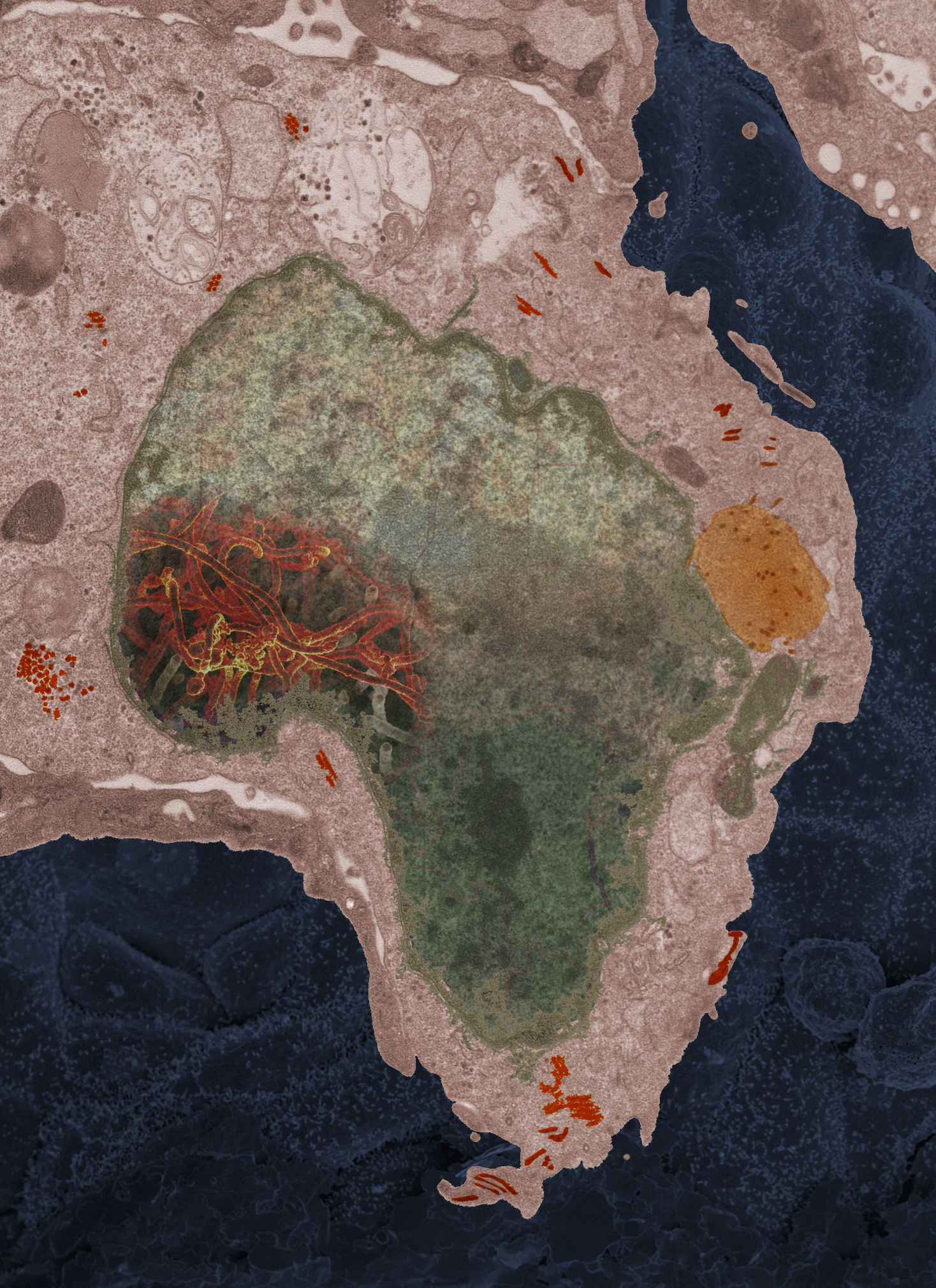
“This one particular cell, it looked like the continent of Africa,” Fischer recalls. “It was a very powerful moment. You see that virus growing in there, it takes you back around to not only the lab work we do, but that there’s an impact on human health.”
Despite viruses’s deadly nature, she still appreciates the “beautiful symmetry in many of them,” she says: “They’re very elegant, and they’re not malicious in and of themselves. They’re just doing what they do.”
Kaiser Health News (KHN) is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
More Must-Reads from TIME
- Cybersecurity Experts Are Sounding the Alarm on DOGE
- Meet the 2025 Women of the Year
- The Harsh Truth About Disability Inclusion
- Why Do More Young Adults Have Cancer?
- Colman Domingo Leads With Radical Love
- How to Get Better at Doing Things Alone
- Michelle Zauner Stares Down the Darkness
Contact us at letters@time.com