
When the disease plaguing her digestive system was at its worst, Kelly Owens once had to rush to the bathroom 17 separate times in the course of a few hours. By the time she was 25, her crippling case of Crohn’s disease had given her arthritis from her ankles all the way up to her jaw and fingertips. The dozens of drugs she took helped a bit, but the brutal side effects included nausea, fatigue and weight gain. Nights were the worst. On good nights, Owens woke up to excruciating pain and couldn’t fall asleep again, trying in vain to find a comfortable position. On bad nights, the diarrhea and vomiting made her so dehydrated, she needed to be hospitalized. “My body was at war with me,” she says. Worse, the powerful drugs she took were weakening her bones: at 25 years old, she had the frail and weakened skeleton of an 80-year-old. There is no known cure for Crohn’s, an inflammatory bowel disease that affects nearly 800,000 people in the U.S. Available medication provides only temporary relief. Owens, who was diagnosed at age 13, eventually developed resistance to all of the drugs she tried, and in February 2017, she says, her doctors told her, “We are out of [treatments] to try; there is nothing left because you have been on them all.”
Hope for Owens and millions of others experiencing a broad range of previously untreatable, or unsatisfactorily treated, diseases may be near, thanks to a breakthrough that seems more science fiction than medical reality. The remarkable convergence of advances in bioengineering and neurology has resulted in a fast-developing way to treat chronic diseases, known as bioelectronic medicine. These advances allow scientists to identify specific nerves and implant devices that can be activated when needed to stimulate or dial down their activity; that in turn controls cells in organs targeted by those nerves that regulate the body’s many immune and metabolic responses. While some bioelectronic, or electroceutical, therapies already exist to treat conditions such as headaches, certain cases of depression, as well as chronic and sinus pain, the new wave of electricity-based strategies could expand to help people with some of the most widespread chronic diseases in the world, including high blood pressure, arthritis, diabetes, some forms of blindness and even dementia.

For Owens, the new approach has been life-changing. After getting an electrical regulator implanted in her chest, she is now living pain-free for the first time in decades. Two weeks after she received the implant, doctors turned it on to a frequency customized to stimulate a specific nerve at just the right energy level to keep her immune system under control. That evening, she forgot to take her pain medication because she wasn’t in pain.
Such promise is already attracting scores of startups and major drug companies. Even with the still rudimentary efforts at stimulating some of the larger nerves in the body to treat, for example, headaches and chronic pain, financial analysts expect the market to reach $7 billion by 2025. Companies like Abbott already have neuromodulation devices designed to stimulate nerves, approved by the Food and Drug Administration, for treating chronic pain. The potential of the electroceutical field is part of a profound shift in the pharmaceutical- industry, which has long been focused primarily on developing new pills. But as blockbuster drug development has stalled in recent years, established pharmaceutical companies like Glaxo-Smith-Kline see electroceuticals as a way to mine a new source of therapeutic possibility—through nondrug treatments that rely more heavily on device and procedure-based methods, such as gene therapies and the recently approved CAR T-cell treatments for certain cancers. The appeal of these new approaches lies in their ability to bring precise and personalized treatment to patients like Owens. Drugs that are taken by mouth end up in nearly every cell in the body and eventually make their way to their intended target, which dilutes their effectiveness and increases the chances they can cause adverse reactions in tissues where they aren’t supposed to be. The pharma industry and patients are eager for more customized approaches. Drug developers are capitalizing on genetic information that can help them better match the right therapies to the right patients—especially for cancer treatments where specially designed drugs are chosen to home in on particular mutations in tumor cells. Isolating certain nerves to stimulate or inhibit represents another promising extension of that bespoke focus.
“There has been frustration that for many diseases for which we make new drugs, there hasn’t been tremendous progress,” says Dr. Brian Litt, professor of neurology and director of the Penn Epilepsy Center at the University of Pennsylvania. If more of the chronic diseases that continue to command the most prescriptions and health care services can be treated with bioelectronic approaches, the market for the field could approach $40 billion. Electroceuticals “are the next wave of new treatments we will have to treat disease,” says Kris Famm, president of Galvani Bioelectronics, a biotech collaboration between Glaxo-Smith-Kline and Google’s Verily that is focused on developing electricity-based therapies.

The idea of tapping into the body’s electrical network is centuries old. In the late 1700s, Italian scientist Luigi Galvani was walking through an open market during a lightning storm when he noticed that frog legs for sale were still twitching. Intrigued, he conducted among the first studies of electrical stimulation, using an electrode to pass a current through a frog leg and observing that the signal prompted the muscles to move.
It turns out that many cellular functions—producing hormones, for example, or contracting or expanding muscles—are regulated by electrical signals that pass through nerves between the brain and the organs where the cells are located. The frequency of those currents determines how active the cells are in performing their assigned function.
Medicine’s attempts to exploit this system grew more refined with time. The earliest were as likely to be hit or miss. In the 1930s, nerves in the brain were stimulated to understand and alleviate some of the symptoms of epilepsy. Electroconvulsive therapy destroyed or compromised nerves to address psychiatric disorders such as schizophrenia and bipolar. In recent decades, with better understanding of how electrical signals work in the body, more effective bioelectronic devices focused on refined modulation of electrical signals—including pacemakers for the heart, cochlear implants, as well as devices to control urinary incontinence and strategies for helping paralyzed muscles to move—have made it to market.
As researchers have learned more about how cells communicate electronically with one another, they are fueling a more sophisticated surge in bioelectronic devices that is delving deeper into more complicated neural networks. Innovations in engineering that are packing chips and other electronic components into tinier and tinier kits to implant in the body, with more power to communicate, charge, stimulate and record, are also expanding the range of diseases that might be treated with a bioelectronic therapy.
Owens could be at the vanguard of a new generation of patients who no longer have to treat chronic conditions by relying on pills that provide temporary and often unsatisfactory relief while exposing them to side effects. In the not too distant future, for example, scientists anticipate that patients with rheumatoid arthritis will no longer suffer from excruciating pain in their joints, but may be able to turn on an implanted electrical device to quiet the immune response that drives their painful inflammation. Or someone with high blood pressure could get an electrical device that would control how well the kidneys filter fluids, alleviating the need to pop pills every day. Or a diabetic could avoid the constant cycle of blood checks and pills or insulin shots, with an electroceutical device at the pancreas that protects their insulin-producing cells. At Massachusetts General Hospital, researchers are working on ways to activate nerves in the eye to restore vision in people with retinal disease, while scientists at Johns Hopkins are convinced that manipulating electrical signals in the brain in just the right way could address conditions from depression to dementia.
That’s the vision of the future promised by electroceuticals. Nerves in the body that regulate specific organs—really specific cells in those organs—could be controlled with the precision of an orchestra conductor calling on specific instruments to generate just the right harmony. “The nervous system really uses electricity as its language,” says Robert Kirsch, chair of biomedical engineering at Case Western Reserve University and executive director of the Cleveland FES Center. “So electrical stimulation can be used theoretically just about anywhere in the nervous system. We need to learn how to speak that language.”
For now, researchers are beginning that decoding project with well-defined nerve systems. For example, the biotech company Neuros Medical, based in Cleveland, is targeting the nerve trunk that runs along a person’s legs as a way to potentially treat phantom pain in amputees. These neural thoroughfares make up a relatively simple network, extending the entire length of the limb. After an amputation, the nerve continues to grow, sending out new extensions that, with nowhere to target, begin to clump into a mass of tangled—and painful—nerve endings called a neuroma.
Zi-Ping Fang, chief scientific officer at Neuros, developed a potential solution for treating such pain. The device includes a surgically implanted electrode that wraps around one or two nerves in the leg. The electrode is connected to a waveform generator implanted in the abdomen that produces a high–frequency current whenever a patient presses a button on a remote control, before shutting off automatically after 30 minutes.
Each time the patient activates the device, it produces a preset current of energy that blocks the pain signals sent by the nerve to the brain. Fang initially thought the relief would be temporary, lasting only as long as the device was activated. But surprisingly, patients in the first pilot study reported feeling pain-free for hours and even days after a treatment session. The researchers still don’t fully understand why, but, Fang says, they hypothesize that besides directly blocking the pain, the electrical therapy may also help to desensitize the nervous system to the pain sensation. “If we give the patients 30 minutes of pain-free time, clinically, some doctors call that a ‘pain vacation.’ It’s not a cure for pain, but for many people in our pilot study they were able to significantly reduce or quit their use of narcotics and improve their quality of life.” The company is now expanding its study beyond the first 10 patients with amputation-related pain to include 180 people in order to further test the device for safety and efficacy.
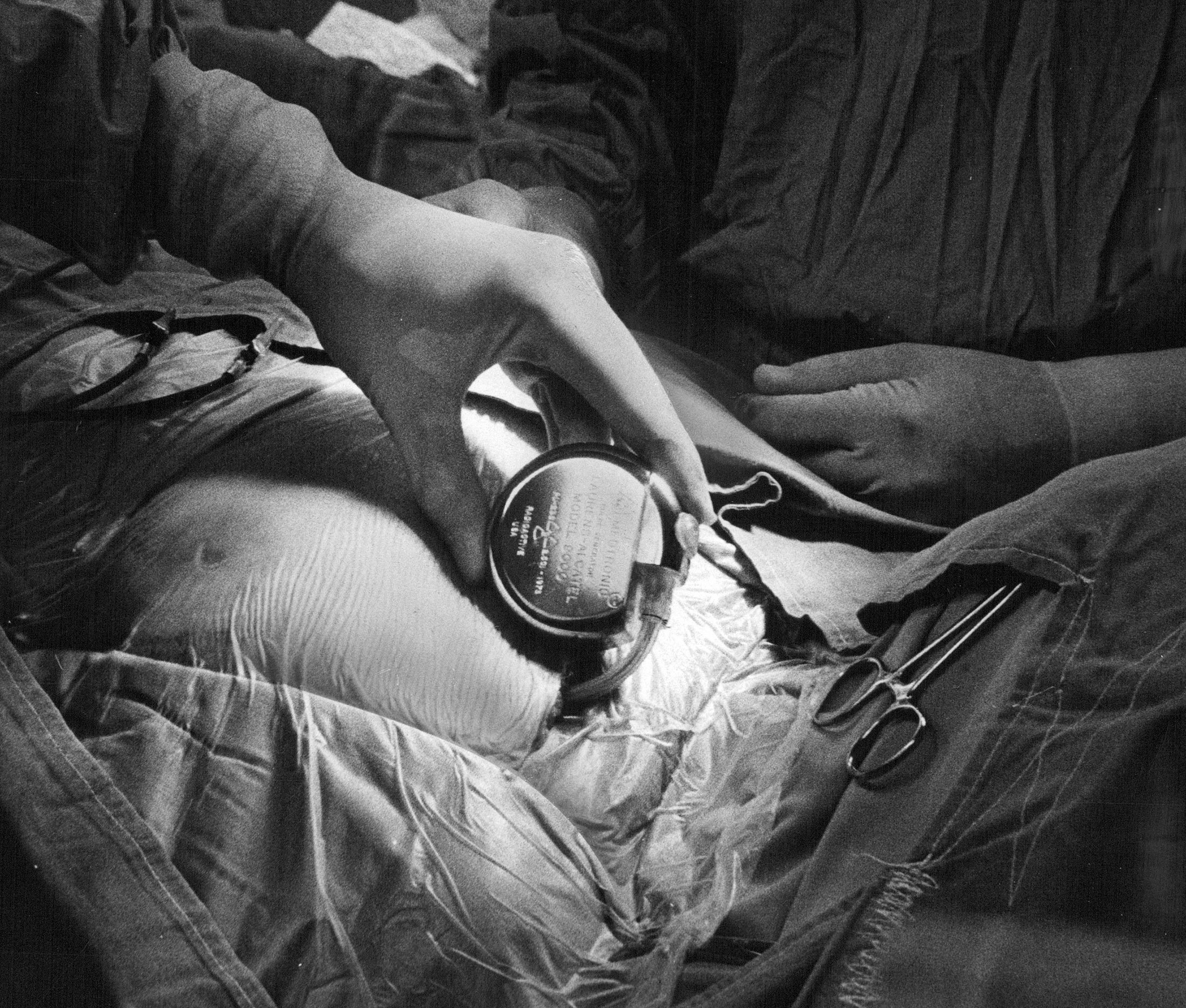
Other companies, like SetPoint Medical, which conducted Owens’ trial, are focusing on the vagus nerve. Named after the Latin word for wandering, the vagus is rooted in the brain stem and branches into the neck, chest and abdomen. It controls everything from sensory functions to swallowing, digestion, respiration and heart rate. Scientists are taking advantage of the fact that the vagus serves as something like a volume control for the nervous system, and because of the relative ease in accessing the nerve—it’s the longest one in the body extending from the brain—it’s an obvious target for those eager to wade into the world of electrical stimulation. But researchers are treading carefully to ensure they trace the vagus’ myriad fringelike connections to the right tissue and the right function. While it starts out as a discrete trunk, the vagus, like many of the other large neural networks in the body, eventually dwindles into brushlike bundles of nerve endings that tap into different organs, different tissues within those organs, and finally different cells within those tissues. “It’s like trying to make a telephone call by putting the call over every single line that is available,” says Kirsch. “It goes to the right line, but it goes to all the other places too.”
There’s more. These connections are piled on top of one another at the tissue level, a chaotic jumble of nerves and nerve endings that are nearly impossible to tease apart. So in trying to trace one fiber, doctors may end up disturbing others, triggering unwanted side effects. The metaphor used by Kip Ludwig, associate professor of biomedical engineering at the University of Wisconsin, is of playing the piano not with your fingers but with your forearms.
So scientists are working on a better road map, building a detailed picture of the major nerve networks in the body. The project, called SPARC, is funded by the National Institutes of Health and aims to map out every nerve of the human nervous system outside the brain. That could illuminate new ways to manipulate electrical signals to control cells connected to those nerves—including what they make and how active they are. Researchers at universities across the country are assigned different major organ systems, and the resulting nerve map will be available to any scientist interested in finding ways to tap into those neural networks to develop a potential electroceutical treatment.
But a neural GPS is only one part of an effective electroceutical. In order to control signals in the right nerves near the organ in question with the right patterns, a device needs to be small enough to be implanted and interface with the target nerve, remain there safely for decades, and be powerful enough to modulate the flood of electrical chatter occurring along that neural circuit. It also needs to communicate with external devices that the patient and doctor use to control the therapy. At Galvani, Famm’s team has spent the past three years designing and building such a system, which he hopes will serve as a platform for applying in a range of different chronic diseases. Within the next couple of years, he says, it will be ready for its first safety and efficacy tests in human patients. “We are more confident than ever that this is possible,” Famm says. “What is beautiful about electroceuticals is that they can get on a nerve right by the organ you are interested in, and it has exquisite potential for precision.”
Kyrana Tsapkini, assistant professor of neurology at Johns Hopkins, is relying on that ability to target nerves to tap into complex functions of the brain, from language to memory. For the past decade, she and her team have been building one of the world’s largest databases on the ways electrical stimulation can affect a variety of neurodegenerative disorders, and the results are already encouraging. In a study of 36 people with Alzheimer’s disease, those who received electrical stimulation showed improvement in their ability to remember words, compared with people who did not get the treatment. Tsapkini is building a database of patients with not just Alzheimer’s but also other neurodegenerative disorders to get a better sense of who might benefit most from a bioelectronic strategy to keep their cognitive functions intact.
For patients like Owens, the early results have been transformative, and she hopes her experience as one of the first to test her device changes the way diseases like hers are treated. Desperate for more options after she’d exhausted the available treatments, she was scouring Facebook for any advice about new therapies when she came across a video interview with Dr. Kevin Tracey, a neurosurgeon at the Feinstein Institute for Medical Research in Manhasset, N.Y. It was 2017, and he had just published his discovery that the body’s inflammatory response was regulated by the vagus nerve. Tracey had founded SetPoint Medical to test the idea that manipulating the electrical signals running along the vagus could control inflammation in auto-immune disorders like Crohn’s.
Since Crohn’s is caused by an overactive inflammatory response in the gut, the goal is to inhibit that inflammation by dialing down the electrical impulses zipping between immune cells around the gut so that the inflammatory response dies down and aggravated gut tissue can start to heal, leading to fewer symptoms and less pain.
Though intriguing, this idea was still an untested theory. But Owens figured it was worth a try. The therapy was not being tested in the U.S., so she and her husband started a GoFundMe campaign to raise money to join SetPoint’s first trials to treat Crohn’s in Amsterdam.
Owens is now in her second year of clinical remission. She no longer takes any medications for her Crohn’s disease and has gone from needing her husband’s help to put on deodorant and button a shirt to working out regularly at the gym and going on long runs. She is back to work as director of education and outreach at the Feinstein Institute, helping patients like her learn more about new therapies such as bioelectronic therapy for treating their autoimmune diseases. Her latest colonoscopy showed that half of the damaged tissue in her colon had healed; without the constant barrage from the immune system, her digestive system is gradually recovering and functioning the way it should. “Now my body just works and I don’t have to think about using it; it just does what it’s supposed to do,” Owens says. “That’s still mind-blowing for me.”
She now turns on the regulator in her chest for only five minutes in the morning and five minutes before going to bed. She started with four sessions of electrical stimulation a day, but found herself forgetting the ones at noon and dinnertime and realized she didn’t need them. She’s aware that the technology is still nascent and still has to prove itself in more trials. But to anyone who might hear that and become skeptical that electroceutical treatments can actually work, she says, “Patients are just really eager to have a new option. And if it’s a placebo effect, all I can say is that it’s a hell of a placebo.”
More Must-Reads from TIME
- Cybersecurity Experts Are Sounding the Alarm on DOGE
- Meet the 2025 Women of the Year
- The Harsh Truth About Disability Inclusion
- Why Do More Young Adults Have Cancer?
- Colman Domingo Leads With Radical Love
- How to Get Better at Doing Things Alone
- Michelle Zauner Stares Down the Darkness
Contact us at letters@time.com