A potentially “life-threatening” labeling mix-up spurred a nationwide recall of a high blood pressure medication, the Food and Drug Administration (FDA) announced.
Accord Healthcare is voluntarily recalling a single lot of 12.5-milligram hydrochlorothiazide tablets, after a pharmacy reported finding a 100-count bottle that actually contained spironolactone tablets, according to the FDA. The rest of the lot (PW05264) has been recalled due to the “potential mix-up of labeling,” but Accord says the remainder of its drugs are unaffected.
Both hydrochlorothiazide and spironolactone can be used to treat high blood pressure, but because spironolactone causes the body to flush out excess water and sodium and store potassium, the FDA warns that patients who mistakenly take it instead of hydrochlorothiazide could experience hyperkalemia, or elevated potassium levels. The effects of hyperkalemia, according to the FDA, range from “limited health consequences to life-threatening situations in certain individuals.”
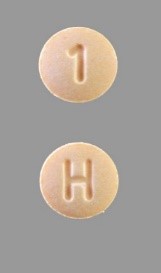
No patients have reported adverse effects in connection with the recall, the FDA says. Those who are prescribed Accord’s hydrochlorothiazide tablets should make sure that the pills are a light orange color with a letter H on one side, and a number 1 on the other. Consumers are urged to report any drugs that do not match that description, or check with their pharmacist.
More Must-Reads from TIME
- Donald Trump Is TIME's 2024 Person of the Year
- Why We Chose Trump as Person of the Year
- Is Intermittent Fasting Good or Bad for You?
- The 100 Must-Read Books of 2024
- The 20 Best Christmas TV Episodes
- Column: If Optimism Feels Ridiculous Now, Try Hope
- The Future of Climate Action Is Trade Policy
- Merle Bombardieri Is Helping People Make the Baby Decision
Write to Jamie Ducharme at jamie.ducharme@time.com