A pharmaceutical company has recalled widely-distributed birth control pills for a packaging error that the Food and Drug Administration (FDA) warns, if taken, could lead to an unintended pregnancy.
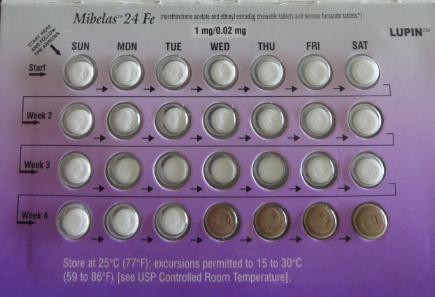
Lupin Pharmaceuticals announced a recall of the chewable pills, sold under the name Mibelas 24 F-E, on May 29. The pills were packaged in a way that reversed the tablets’ order, meaning that the first four pills were non-hormonal placebos rather than the active tablets they were supposed to be.
“As a result of this packaging error, oral contraceptive tablets that are taken out of sequence may place the user at risk for contraceptive failure and unintended pregnancy,” the FDA’s statement reads.
The FDA warns that the packaging error also means taking the pills could cause “significant” harm to both maternal and fetal health. However, at the time of writing, no such adverse events have been reported.
The pills, which are packaged in blister packs containing 28 tablets, were distributed nationwide in the U.S. to wholesalers, clinics and retail pharmacies. Consumers who have the product are advised to return the product to the pharmacy or place of purchase, as well as contact their healthcare provider if they have experienced any adverse effects.
Consumers who have questions about the recall can call the manufacturer, Lupin Pharmaceuticals, at 800-399-2561.
More Must-Reads from TIME
- Cybersecurity Experts Are Sounding the Alarm on DOGE
- Meet the 2025 Women of the Year
- The Harsh Truth About Disability Inclusion
- Why Do More Young Adults Have Cancer?
- Colman Domingo Leads With Radical Love
- How to Get Better at Doing Things Alone
- Michelle Zauner Stares Down the Darkness
Write to Kate Samuelson at kate.samuelson@time.com