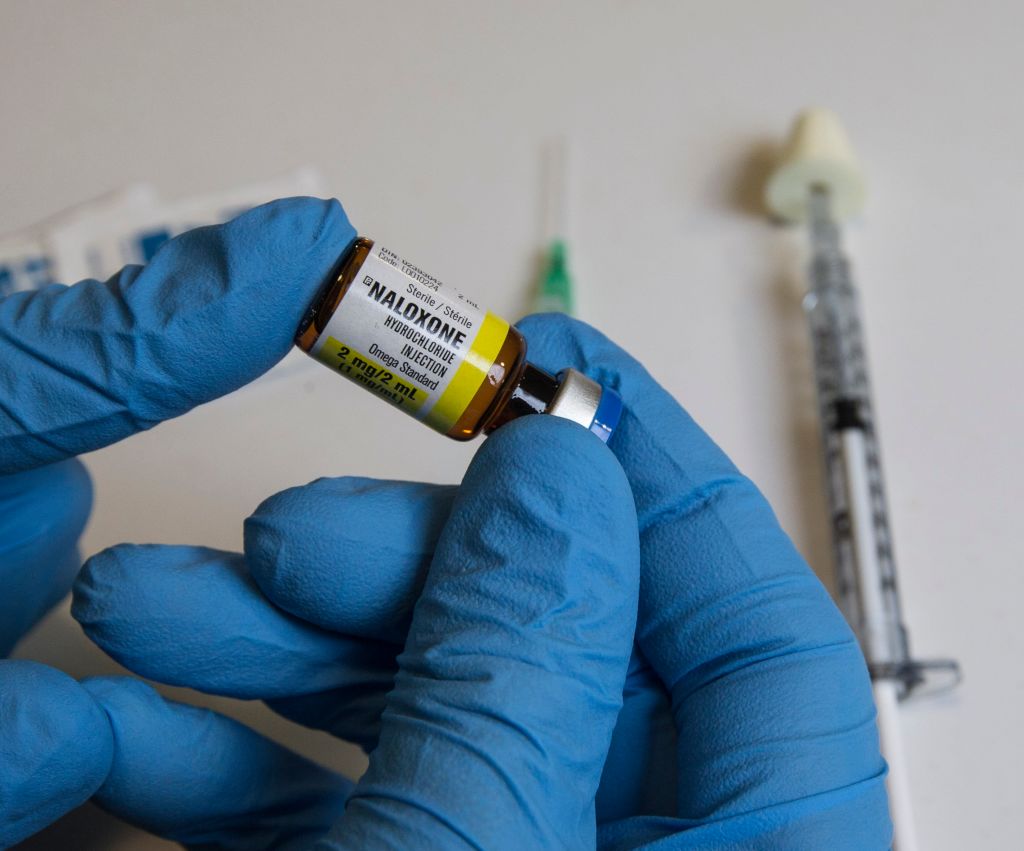
(Bloomberg) — The Food and Drug Administration is trying to make it easier for drug companies to make nonprescription versions of opioid overdose antidote naloxone.
The agency has created sample consumer-friendly labels drugmakers can use when they apply for permission to sell over-the-counter naloxone as a nasal spray or auto-injector, FDA Commissioner Scott Gottlieb said in a statement on Thursday. This is the first time the agency has created and tested a label for a drug consumers can purchase directly, he said.
Key Insights
Emergent BioSolutions Inc. sells a prescription naloxone nasal spray known as Narcan. Opiant Pharmaceuticals Inc. developed the spray and collects royalties on it. Kaleo Inc. markets prescription Evzio, a naloxone auto-injector. Most states allow naloxone to be dispensed without a prescription but pharmacist participation has been spotty. Gottlieb said a requirement for testing showing that consumers can read a label and use a nonprescription drug properly has been a barrier for companies that want to sell over-the-counter naloxone. Companies will still have to ensure consumers understand parts of the label that are product-specific.
Get More
The move is part of a wider effort to reduce overdose deaths. The FDA has explored limiting the number of opioids per prescription. It has also required more training for medical professionals on prescribing the painkillers. Opioids were involved in 47,600 overdose deaths in 2017, or almost 68 percent of all fatal overdoses, according to the Centers for Disease Control and Prevention.
More Must-Reads from TIME
- Why Trump’s Message Worked on Latino Men
- What Trump’s Win Could Mean for Housing
- The 100 Must-Read Books of 2024
- Sleep Doctors Share the 1 Tip That’s Changed Their Lives
- Column: Let’s Bring Back Romance
- What It’s Like to Have Long COVID As a Kid
- FX’s Say Nothing Is the Must-Watch Political Thriller of 2024
- Merle Bombardieri Is Helping People Make the Baby Decision
Contact us at letters@time.com