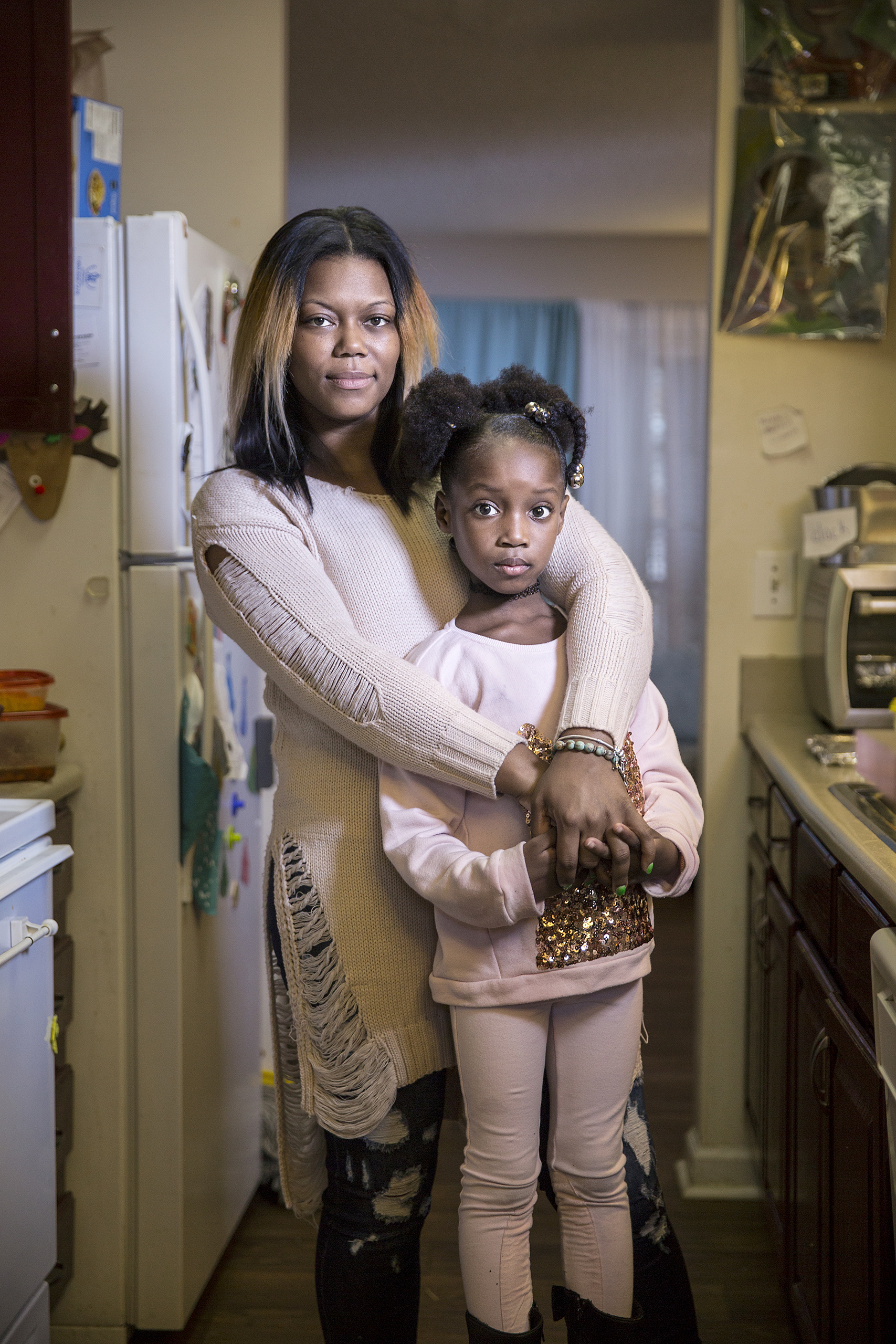
In the fall of 2010, Masonia Traylor’s life changed overnight. Then 23, she was living with her new boyfriend in Atlanta and working as a pharmacy technician at a nearby drugstore. She took good care of herself, always went for an annual checkup, and ever since she graduated from high school, she had made it a practice to get tested for HIV and other sexually transmitted diseases. Every year, her tests would come back negative. Until one didn’t.
“At first I felt like the test was a lie, that the labs got mixed up,” she says. “Then I started to feel guilt. I felt like I was being punished for not waiting to have sex until I was married.”
It was October when her doctor told her she had tested positive for HIV. For the next several weeks, she tried to digest the news, sometimes crying uncontrollably, sometimes numb. But she also had treatment options to sort out, so by early November, she was back at her doctor’s office. That’s when the doctor delivered another shock: Traylor was pregnant.
Her first reaction was similar to the one she’d had with her HIV diagnosis: disbelief. Then her mind began reeling. The idea of bringing an HIV-positive baby into the world was unthinkable. “I wasn’t sure if I was going to keep the baby or not,” she says.
At her next appointments, her doctor walked her through the possibilities. Traylor learned that she could take drugs that were safe for her and the baby and would all but eliminate the risk of passing on her infection. As long as she agreed to take these anti-HIV medications throughout her pregnancy, and give her baby similar ones for a few weeks after birth, it was very unlikely the child would ever be HIV-positive. That’s ultimately what Traylor chose to do, and her daughter Marissa is now 5–and HIV-free.
It’s thanks to these medications–a cocktail built around the antiviral drug zidovudine, also called AZT–that rates of so-called perinatal transmission of HIV are so low in some parts of the world. In the U.S., fewer than 100 HIV-positive babies were diagnosed in 2015, representing a remarkable success story in the history of an epidemic that has killed 35 million.
In fact, newborns were the first group of people exposed to infected blood and successfully protected with drugs from contracting HIV. And today those drugs have brought mother-to-baby transmission rates to under 2% in the U.S.
Experts agree that major progress has been made. About 150,000 babies are diagnosed with HIV each year around the world, down from a peak of more than 600,000 per year in the 1990s. “The idea of eliminating mother-to-child transmission of HIV is officially on the table,” says Dr. Deborah Cohan, a professor of obstetrics and gynecology and the director of the Perinatal HIV Clinic at the University of California, San Francisco (UCSF). The success is owed largely to the greater funding for and availability of HIV testing and antiviral drugs.
But do the math and it’s clear that even among some groups in the U.S., and also elsewhere around the world, there are blemishes on the rosy picture. Globally, more than 400 babies are born every day with the virus, and the vast majority of them, if left untreated, will die before their fifth birthday.
The key to reducing that number isn’t simply making new scientific advances in medicine–the drugs that can prevent HIV transmission exist, and they work well. Instead, the challenge is one of access: getting women tested so that they know their HIV status in the first place, and then following up with them to make sure they’re getting–and taking–those infection-blocking drugs.
A growing number of HIV experts agree that getting this right doesn’t just hold promise for expectant moms and their babies. It could also help reduce the spread of the epidemic as a whole.
The model for preventing infection from mothers to newborns provides a test case on how to stem HIV transmission writ large. If women, properly treated, can end up with HIV levels so low that they can’t infect the babies they share blood with in utero, then it stands to reason that adults who keep their viral levels down won’t be able to transmit HIV either.
That has already proved true in study after study; when taking the right drugs, people with HIV almost never pass on the virus. Still, even between mothers and babies, who benefit the most from this understanding, there are significant barriers to ensuring that everything goes exactly according to that plan.
“I wasn’t sure the medicine was going to work,” Traylor says. “If my blood had HIV in it, I didn’t understand how it would not transmit to the baby.” It didn’t matter that Traylor’s doctor had assured her that treating her own infection meant her child could be HIV-free. She was skeptical. She also worried about what harm the drugs might do to the developing fetus. “I had to find some level of trust in the universe to be able to do it,” she says of starting her HIV therapy.
Or some trust in the science. “We have the drugs to prevent mother-to-child transmission,” says Dr. Katherine Luzuriaga, a professor of molecular medicine at the University of Massachusetts. “We know that anti-HIV treatment is remarkably effective in preventing transmission. The issue becomes one of implementation.”
Central to that challenge are incorrect but stubbornly held judgments about the virus, like who gets it and why. Stigma is an especially persistent barrier to women seeking testing, not to mention filling prescriptions for and taking drug treatments. Traylor, who knew that getting an annual HIV test was a smart thing to do, never thought she would end up positive. She figured that was something that happened to other kinds of people.
When Traylor first brought her medications home, she peeled off the labels so that no one other than her boyfriend would see the drug names and Google them. She also never brought home any information or brochures on HIV from the doctor’s office. “I had internalized the self-inflicted stigma,” she says.
Traylor’s behavior is far from unusual. At UCSF, pharmacists have put HIV pills in special unmarked blister packs so that they look like vitamins, and some women have poured their pills into bottles used to hold prenatals. To the doctors treating these women, these acts of discretion seem like a minuscule trade-off for ensuring that their patients continue to swallow the medications they need daily–even if it may reinforce the shame and stigma that can come with an HIV diagnosis.
The cost of the drugs, which run into the tens of thousands of dollars on average per year, can also be a deterrent for infected moms. While HIV treatment during pregnancy is covered by Medicaid, that reimbursement isn’t guaranteed for the mother after she delivers. The availability of coverage of antiviral drugs can vary greatly from state to state, and changes to the Affordable Care Act make the future even more uncertain.
These barriers, and the number of babies born with HIV, are extremely frustrating for experts and doctors because there is such clear evidence that it’s possible for HIV-positive women to give birth to HIV-free babies.
In the more than two decades since AZT was first tested on pregnant women, groundbreaking trials have shown that the sooner a pregnant woman who is HIV-positive is treated, the better her chances of delivering a baby who isn’t infected.
But even if expectant moms aren’t diagnosed with HIV until their third trimester, transmission risk can still be lowered dramatically with late treatment. Even during labor there’s still hope; it’s now routine for any HIV-positive mother to get both pill and IV drug treatment to protect the baby from exposure during delivery, and often enough, it works.
In 2010, doctors found that even if nearly everything is stacked against an infant–even if the infant has already contracted the virus in utero–drugs can still drop the newborn’s virus levels to vanishingly low levels.
When a pregnant woman who wasn’t aware that she was HIV-positive arrived at the University of Mississippi to have her baby, pediatrician Dr. Hannah Gay decided on a risky treatment in order to prevent the baby–who had almost certainly contracted HIV in utero–from getting sick from the virus. Instead of waiting a few weeks to confirm the infection, Gay started the baby on an aggressive dose of three HIV drugs within hours of her birth. The idea was to ambush the virus with enough antiviral drugs that the HIV could not embed itself in the baby’s body.
The intensive therapy brought the infant’s viral load down so low that it became undetectable with a blood test. And even after the baby’s mother stopped those antivirals a year and a half later, the most sophisticated tests still couldn’t find traces of HIV for 27 months. It represents the longest HIV remission achieved with drugs on record.
“What came out of that Mississippi case is that starting therapy early can substantially, substantially reduce reservoirs of HIV in the body,” says Dr. Deborah Persaud, a professor of pediatrics at the Johns Hopkins University School of Medicine, who was part of the baby’s care team.
The case provided valuable information about how far doctors can push the limits of remission. It also came with a painful reminder: anti-HIV drugs only work, in the long term, when they’re taken consistently. Shortly after the baby’s seemingly miraculous remission, the infant and her mom stopped attending their doctor visits. When they returned several years later, the toddler’s virus levels were up once again, putting her immune system at risk of crashing and leading to full-blown AIDS. The child has since started on drug treatment again, and today her virus levels are back below detectable levels.
Buoyed by that case, researchers are now refining the strategy that Gay used, testing specific doses and combinations of drugs to optimize their antiviral effect.
Early detection and consistent treatment is the best-case scenario for doctors treating babies with HIV. It’s what the World Health Organization recommends, and that’s in line with the standards of care set by the Centers for Disease Control and Prevention too.
“This is an exciting time for the field,” says Luzuriaga. “We don’t quite have all the answers yet, but they are coming soon.”
And when they do, experts hope that the object lessons learned in the U.S. can lead to bigger drops in mother-to-child transmission in the parts of the world where too many newborns are still infected in utero. Already, inspired by the Mississippi baby, more doctors around the world are starting to treat HIV-positive pregnant women as early as possible, including in the first trimester, even though they believe most infections to the developing baby occur later in pregnancy. And the more women like Traylor show that it’s possible to protect newborns from HIV, the more public-health officials hope the stigma against the disease will crumble.
For her part, Traylor has come a long way from those early, shame-filled days. A few years after her diagnosis, she found out that a friend of hers had died of complications from HIV, which he’d kept a secret just like she had.
“He gave up and stopped taking his meds,” she says. “I wonder if he knew I had it too, that he wasn’t alone, maybe he wouldn’t have given up.”
Seeing her daughter now, Traylor knows she made the right choice.
More Must-Reads From TIME
- The 100 Most Influential People of 2024
- Coco Gauff Is Playing for Herself Now
- Scenes From Pro-Palestinian Encampments Across U.S. Universities
- 6 Compliments That Land Every Time
- If You're Dating Right Now , You're Brave: Column
- The AI That Could Heal a Divided Internet
- Fallout Is a Brilliant Model for the Future of Video Game Adaptations
- Want Weekly Recs on What to Watch, Read, and More? Sign Up for Worth Your Time
Contact us at letters@time.com