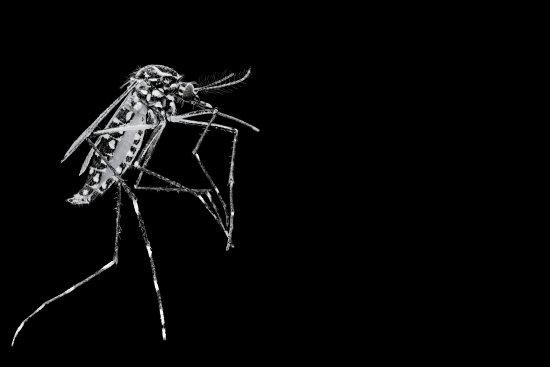
When a mosquito lands on a patch of exposed skin and unsheathes a needle-like proboscis to pierce a blood vessel, she–and it is always a she–is not just sucking up a blood meal. She’s also leaving something behind with her saliva. In some cases, it’s a harmless anticoagulant that keeps the blood flowing and causes nothing more than an itchy welt.
But in many parts of the world, different species of the mosquito carry serious and sometimes deadly diseases. Beyond the Zika virus, carried by the Aedes aegypti, there is malaria, borne by the Anopheles genus of mosquito. There are mosquitoes on every continent save Antarctica that carry dengue and others that bear West Nile virus, Japanese encephalitis and a host of other diseases. Hundreds of millions of people will be infected by mosquitoes this year, and more than a million will die. “The mosquito is the most efficient transmitter of disease in the animal kingdom,” says Grayson Brown, director of the University of Kentucky’s Public Health Entomology Laboratory. “They are responsible for more deaths than any other form of insect”–or any other animal, period.
After years of progress, it’s a war we’re beginning to lose. The effectiveness of the insecticides and other tools that once stopped mosquitoes is dwindling, with experts estimating that insecticide-treated bed nets–long the gold standard of mosquito control in hard-hit developing countries–could lose much of their effectiveness by 2020. At the same time, urbanization and climate change are allowing the insects to expand their range–including to developed countries like the U.S.
The good news is that scientists are developing next-generation technologies that enable them to hijack mosquitoes’ genetic code and reproductive systems. Some stop mosquitoes from transmitting disease; others radically reduce populations without pesticides that come with environmental side effects. The gene-editing technique known as CRISPR-Cas9, which can do both, could potentially be used to render certain disease-carrying species extinct.
The notion of tampering with the genes of an entire species inevitably worries bioethicists, but many experts believe that mosquito-borne diseases are so dangerous that we have no choice but to try–at least in the most vulnerable countries with the least effective control systems. New drugs and insecticides can take up to 10 years and hundreds of millions of dollars to develop, and in the meantime, millions of people will die.”We don’t have that kind of time,” says Dr. Richard Kamwi, Namibia’s former Health Minister who now coordinates public-health efforts to eliminate malaria across southern Africa. “A malaria vaccine has been 10 years down the line for the past 25 years. We need something now, before the tools we do have stop working.”
New genetic controls, Kamwi argues, have to be part of the solution–and by that he means eradication. “The mosquito vectors must be eliminated to eliminate the parasites,” he says. “I want to call on all the researchers and say that where they have been walking, they must start running. Where they have been jogging, they must start sprinting.”
In rooms artificially heated and humidified to resemble the mosquito-friendly tropics, researchers in the entomology laboratory at Imperial College London are trying to carve an epitaph for disease-carrying mosquitoes. Chryssa Taxiarchi, a 25-year-old Ph.D. candidate, plucks a handful of mosquito larvae out of a plastic water-filled tray and places them in a petri dish. They are Anopheles gambiae, the species responsible for nearly 90% of malaria cases in the world. Under a microscope, the wriggling, millimeter-long larvae glow red, proof that painstaking years-long experimentation with injecting mosquito embryos with specially designed DNA is starting to work.
These mosquitoes, when adult, will produce only male offspring–and since only female mosquitoes bite, Imperial College researchers hope that they can essentially instill evolutionary femicide into the insects’ DNA. When released into the wild, these mosquitoes (or their descendants) will seek out mates and breed more male mosquitoes until there are no females left in the area to lay eggs. “It is edgy science,” says researcher Sara Rosas Martins as she collects mosquito eggs from mesh-covered cages. “If it works, it will have a major impact.”
Austin Burt, a professor of evolutionary genetics at Imperial College and the developer of the technology, didn’t set out to commit mosquito genocide. “Our target is malaria, not mosquitoes,” he says. “Mosquitoes are a means to an end.” But once unleashed, Burt’s mosquitoes have no kill switch. They will carry out their mission until there are no females left. To some experts, it’s a small sacrifice. But others worry about the implications of leaving a biological niche empty.
That concern is part of what drove Anthony James, a molecular biologist at the University of California, Irvine, to take a different tack. He’s working to make mosquitoes incapable of carrying malaria and, eventually, other pathogens like Zika. This technique leaves the mosquitoes in place while disarming them. “Nobody likes mosquitoes, but you can live with them if they are not giving you disease,” he says. “Better to fix the ones you have than deal with whoever comes along next.”
Like Burt, James is using gene drive, a process of genetic manipulation that ensures that a selected trait will be passed on to nearly all offspring, not just half, as happens in most cases of natural inheritance. Think of it as a postal service for genes, in which a packet of instructions–the new DNA–is delivered to a specific address on the genome that tells the mosquito to destroy the malaria parasite and to forward those directions to descendants.
But malaria is likely as old as the human population that hosts it, and just as it has evolved resistance to every new drug used against it, some think the parasite could find a way around this genetic tinkering. “Mosquitoes are full of surprises,” says Dan Strickman, senior program officer for vector control at the Bill & Melinda Gates Foundation. The foundation has supported James’ research and has also invested $40 million in Burt’s work on mosquito eradication.
Evolution takes time, though, and like the smallpox virus, which was finally eradicated in 1980, the malaria parasites that infect humans need hosts to survive. If no humans are getting sick–and no mosquitoes are spreading the parasite–malaria could die out like smallpox.
Both Burt and James believe that their genetically modified (GM) mosquitoes could be ready much more quickly than drugs or vaccines, which have long been the tools of choice for health groups. But the phase trials needed to test the technology in the field will still take time–and that’s assuming they can overcome a deep public skepticism in much of the world toward GM animals. It took GM salmon 20 years to pass regulations in the U.S., and it still hasn’t reached the market. “Is it right for us to intentionally eliminate an entire species?” asks bioethicist and Stanford law professor Henry Greely. “In the U.S., we have the Endangered Species Act, which tells us not to do that.”
Even in Brazil, where Zika has infected at least 1.5 million people, there is discomfort with a promising GM-mosquito technology. A pilot program run by the U.K.-based biotechnology company Oxitec floods an area with male mosquitoes genetically engineered to pass a fatal gene to its offspring. Those males mate with wild females, precipitating a population crash when the offspring die before maturity. In the southeastern Brazilian city of Piracicaba, Oxitec has released more than 44 million males since April 2015, reducing the population of wild A. aegypti larvae by 82%.
But that progress is in danger. In January, local news organizations and anti-GM activists posited a false correlation between Oxitec’s mosquito releases and the Zika outbreaks. As a result, Brazilian authorities are delaying the planned scale-up of a program that could help quell the spread of Zika and diseases like dengue, which infected 1.6 million Brazilians last year. A Change.org petition signed by more than 160,000 people has delayed a similar Oxitec trial in the Florida Keys despite fears about Zika. “We are very concerned that we don’t really know what will happen if you put a genetically modified mosquito into our local environment,” says environmental activist and Florida Keys resident Michael Welber.
For all the angst, the Oxitec intervention is much less permanent than Burt’s or James’ technology. While the program uses mosquitoes that are genetically modified to pass on a killer gene, it is not a trait that can be passed on through subsequent generations, since the offspring die before they can mate. Instead, the mosquitoes have to be released by the tens of thousands on a weekly or twice-weekly basis to ensure that enough modified males mate with wild females. For a city, that costs $7 a person per year, on average–doable for a middle-income nation like Brazil but out of reach for the extremely poor African nations where the bulk of deaths from mosquito-borne disease occur. By contrast, Burt estimates that he would need to release a one-off “bucket or two” of GM mosquitoes–about 400–per small village to create an enduring effect. That’s why the Gates Foundation is pursuing gene-drive tech instead of the simpler, and potentially less controversial, Oxitec solution.
The specter of unintended consequences inevitably haunts every discussion about using GM mosquitoes or even eliminating certain species. “There is a potential that we are in trouble if all mosquitoes are gone,” says Cameron Webb, a medical entomologist at the University of Sydney in Australia. Mosquitoes are an abundant snack for many kinds of birds, bats, fish and frogs, and they may also play an important role as pollinators for some plants. Still, he says, the selective elimination of a species like the Zika-carrying A. aegypti is not likely to do much harm, especially since it is largely an urban-dwelling creature. “It’s probably not a very useful food source for whatever animals may be chasing insects around our cities,” says Webb. “If you were to eradicate A. aegypti, the ecological consequences are probably going to be quite low, and I think that is a fair trade-off given the incredible reduction in mosquito-borne diseases.”
Eliminating malaria-carrying mosquitoes, on the other hand, might prove more difficult. There are more than 24 species of Anopheles mosquitoes that carry the parasite, and their elimination could have a larger ecological impact. How should that impact be weighed against the health benefits? “If you are confident that you can save 1,000 humans, and it will decimate two species of bats and cause great harm to a particular flowering plant, do you go ahead?” asks bioethicist Greely. “What if it saves 1 million lives?”
Environmental consequences aside, the reality is that it would be hard for any technology to reach every corner of the world where mosquitoes exist. As much as James would love to see a mosquito-free planet, he doubts we will ever get that far. “I just don’t think there are enough resources, enough will, to do this,” he says. “There will always be small, isolated pockets of mosquitoes that will persist.”
Which is why, says the University of Kentucky’s Brown, we can’t afford to let up on the workaday methods that may not offer the promise of total extermination but can still save lives–including clearing mosquito habitats, spraying walls and using bed nets. Gene editing shows promise, but so did insecticides like DDT when they were introduced–and mosquitoes are still here.
–With reporting by TARA JOHN/LONDON and ALEXANDRA SIFFERLIN/PIRACICABA, BRAZIL
