By David Stout
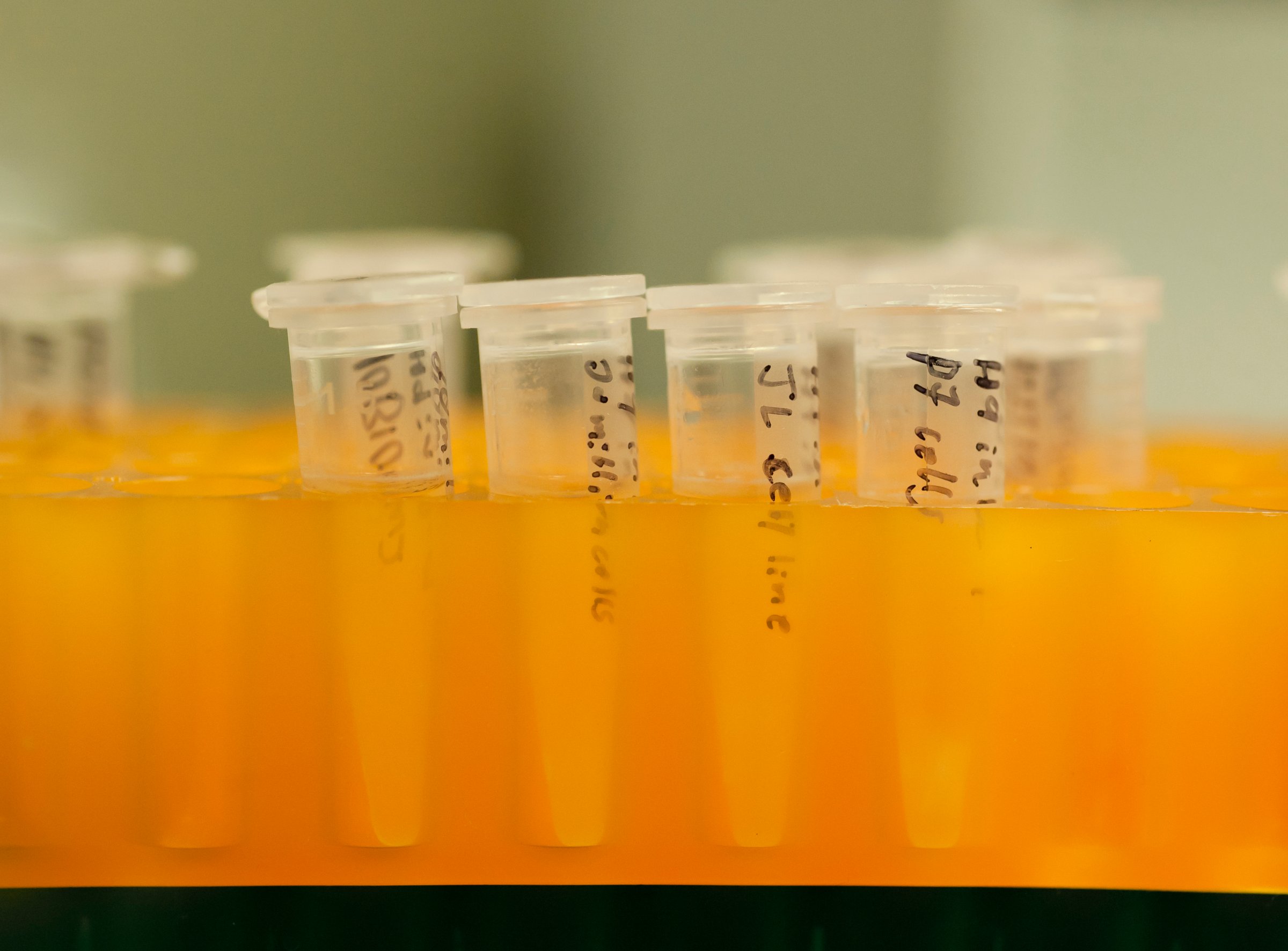
The U.S. Food and Drug Administration has allowed Google-backed company 23andMe to begin marketing a home genetic test for Bloom Syndrome — an inherited condition characterized by shortness of height and increased risk of cancer.
The FDA also announced that it intends to exempt similar genetic testing devices from its premarket review protocol.
Screening tests are largely used by prospective parents who are concerned that their future children may inherent harmful genetic disorders.
“Today’s authorization … along with FDA’s intent to exempt these devices from FDA premarket review, supports innovation and will ultimately benefit consumers” stated spokesman Alberto Gutierrez.
More Must-Reads From TIME
- The 100 Most Influential People of 2024
- The Revolution of Yulia Navalnaya
- 6 Compliments That Land Every Time
- What's the Deal With the Bitcoin Halving?
- If You're Dating Right Now , You're Brave: Column
- The AI That Could Heal a Divided Internet
- Fallout Is a Brilliant Model for the Future of Video Game Adaptations
- Want Weekly Recs on What to Watch, Read, and More? Sign Up for Worth Your Time
Contact us at letters@time.com